 |
|
|||||||||||
|
|
|
|||||||||||||||||||||||||
DownloadsThis is our main download page for ChemSep containing updates for the latest LITE version as well as case stories and examples. This page is changing frequently.
DatabasesOur Databases page provides an overview of all the data we make publically available.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Files | Descriptions |
| [fsd] [png] |
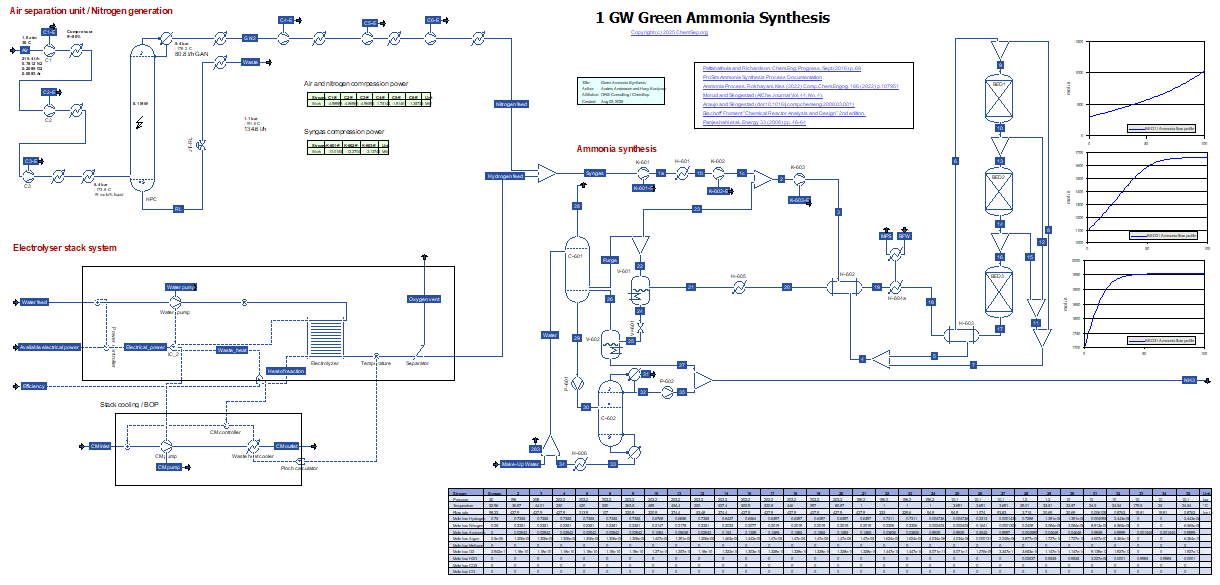 NEW:
Green Ammonia Synthesis (10/11/2025)
Green Ammonia is produced using green hydrogen from 1 GW electricity, air and water. Hydrogen is produced via electrolysis of water and nitrogen is produced via cryogenic air separation.
Ammonia is produced using a three-bed quench / cold-shot process, inspirated by Morud and Skogestad (AIChe Journal Vol. 44, No. 4), Araujo and Skogestad (comp. chem. engng., 2008), and Temkin-Pyzhev kinetics from Bischoff & Froment "Chemical Reactor Analysis and Design" 2nd edition.
The reaction conditions and bed heights mimic those applied in Morud and Skogestad, using a higher inlet temperature/higher degree of pre-heating.
The full ammonia loop is modelled including high-pressure separation of ammonia by cooling.
Ammonia is recovered from purge and flash gas by scrubbing with water and subsequent distillation.
This flowsheet is based on three separate flowsheets that were modified and combined, and it assumes cold sea water is available for cooling.
The air separation has been modified from the original file in order to increase the production of gasseous nitrogen and discarding production of oxygen and argon.
The achieved specific energy requirement for nitrogen generation at 5.3 bara is 0.232 kWh/Nm3 which is in good agreement with cited nitrogen generation efficiencies
(Messer and
TAIYO NIPPON SANSO Technical Report No.41, 2022).
The ammonia refrigeration unit cools the syngas stream to 1 C in order to separate ammonia from the mixture (the refrigeration unit has not been modelled).
The specific energy consumption for ammonia synthesis accounting for nitrogen and syngas compression but excluding nitrogen generation and electrolysis, is 310 kWh/t NH3, also in good agreement with
established data by the Danish Energy Agency).
NEW:
Green Ammonia Synthesis (10/11/2025)
Green Ammonia is produced using green hydrogen from 1 GW electricity, air and water. Hydrogen is produced via electrolysis of water and nitrogen is produced via cryogenic air separation.
Ammonia is produced using a three-bed quench / cold-shot process, inspirated by Morud and Skogestad (AIChe Journal Vol. 44, No. 4), Araujo and Skogestad (comp. chem. engng., 2008), and Temkin-Pyzhev kinetics from Bischoff & Froment "Chemical Reactor Analysis and Design" 2nd edition.
The reaction conditions and bed heights mimic those applied in Morud and Skogestad, using a higher inlet temperature/higher degree of pre-heating.
The full ammonia loop is modelled including high-pressure separation of ammonia by cooling.
Ammonia is recovered from purge and flash gas by scrubbing with water and subsequent distillation.
This flowsheet is based on three separate flowsheets that were modified and combined, and it assumes cold sea water is available for cooling.
The air separation has been modified from the original file in order to increase the production of gasseous nitrogen and discarding production of oxygen and argon.
The achieved specific energy requirement for nitrogen generation at 5.3 bara is 0.232 kWh/Nm3 which is in good agreement with cited nitrogen generation efficiencies
(Messer and
TAIYO NIPPON SANSO Technical Report No.41, 2022).
The ammonia refrigeration unit cools the syngas stream to 1 C in order to separate ammonia from the mixture (the refrigeration unit has not been modelled).
The specific energy consumption for ammonia synthesis accounting for nitrogen and syngas compression but excluding nitrogen generation and electrolysis, is 310 kWh/t NH3, also in good agreement with
established data by the Danish Energy Agency).
|
| [fsd] [png] |
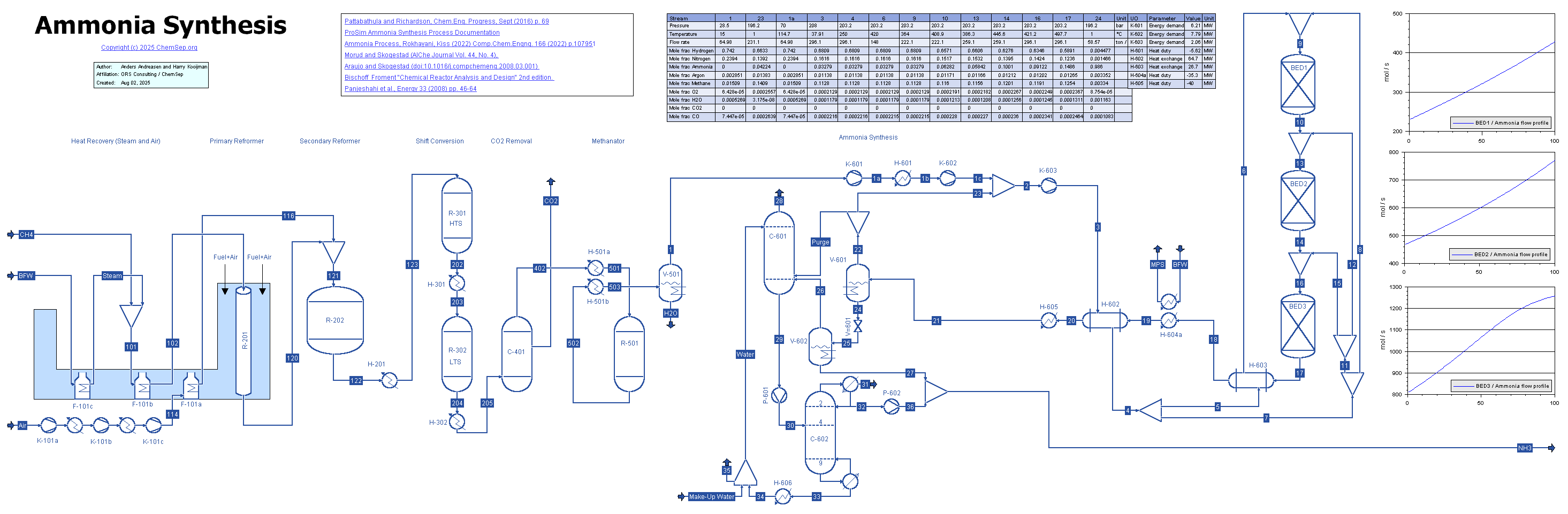 Ammonia Synthesis (2/8/2025)
Simulation of ammonia synthesis with steam reformer, shift reactors, and methanation, followed by Ammonia synthesis using a three-bed quench / cold-shot process with inspiration from
Morud and Skogestad (AIChe Journal Vol. 44, No. 4), Araujo and Skogestad (comp. chem. engng., 2008), and Temkin-Pyzhev kinetics from Bischoff & Froment "Chemical Reactor Analysis and Design" 2nd edition.
The reaction conditions and bed heights mimic those applied in Morud and Skogestad, using a higher inlet temperature/higher degree of pre-heating.
The full ammonia loop is modelled including high-pressure separation of ammonia by cooling and the effect of Argon and Methane impurities.
A colder recovery lowers the reactor inlet concentration at the reactor inlet. Here we obtained 5% ammonia at the inlet and 15% at the outlet.
Note that the steam reformer, shift and methanation reactors are modeled with simple, fixed conversion reactors.
When equilibrium constants are entered proper equilibrium reactors could be used.
Similarly, the CO2 removal unit is modeled using a simple component splitter.
This absorber actually uses MDEA and also involves a regenerator. A more detailed modelling is left as exercize.
Ammonia Synthesis (2/8/2025)
Simulation of ammonia synthesis with steam reformer, shift reactors, and methanation, followed by Ammonia synthesis using a three-bed quench / cold-shot process with inspiration from
Morud and Skogestad (AIChe Journal Vol. 44, No. 4), Araujo and Skogestad (comp. chem. engng., 2008), and Temkin-Pyzhev kinetics from Bischoff & Froment "Chemical Reactor Analysis and Design" 2nd edition.
The reaction conditions and bed heights mimic those applied in Morud and Skogestad, using a higher inlet temperature/higher degree of pre-heating.
The full ammonia loop is modelled including high-pressure separation of ammonia by cooling and the effect of Argon and Methane impurities.
A colder recovery lowers the reactor inlet concentration at the reactor inlet. Here we obtained 5% ammonia at the inlet and 15% at the outlet.
Note that the steam reformer, shift and methanation reactors are modeled with simple, fixed conversion reactors.
When equilibrium constants are entered proper equilibrium reactors could be used.
Similarly, the CO2 removal unit is modeled using a simple component splitter.
This absorber actually uses MDEA and also involves a regenerator. A more detailed modelling is left as exercize.
|
| [fsd] [png] [pdf] |
 Maldistribution Model (23/3/2025) for Oxygen / Argon inspired by Billingham and Lockett. We recreated it from separate distillation columns in the flowsheet as well as from using the ChemSep Parallel Column Model (PCM)).
Billingham and Lockett used a stage-to-stage calculation with a constant relative volatility to model the column sections (they are renamed A and B)
The ChemSep PCM has a liquid distributor tray above the two parallel sections and a gas distributor tray below the parallel section.
These distributor trays are modelled with a stage efficiency set to a very small number such as to avoid numerical issues in the simulation.
A particular advantage of using the ChemSep PCM for these calculations is that any thermodynamic model can be used (including a constant relative volatility model as was used by Billingham and Lockett and here).
Maldistribution is modelled by splitting the liquid flow so that the flow to the left differs from the flow to the right as shown on the flowsheet.
One small difference between our model and theirs is that they did not split the vapor flow as shown in Fig. 11; rather they assumed the gas flow to the base of each column to be the same.
We can obtain the same result by specifying a gas flow to the stream splitter that is twice that needed and a split ratio of 50 percent.
Maldistribution Model (23/3/2025) for Oxygen / Argon inspired by Billingham and Lockett. We recreated it from separate distillation columns in the flowsheet as well as from using the ChemSep Parallel Column Model (PCM)).
Billingham and Lockett used a stage-to-stage calculation with a constant relative volatility to model the column sections (they are renamed A and B)
The ChemSep PCM has a liquid distributor tray above the two parallel sections and a gas distributor tray below the parallel section.
These distributor trays are modelled with a stage efficiency set to a very small number such as to avoid numerical issues in the simulation.
A particular advantage of using the ChemSep PCM for these calculations is that any thermodynamic model can be used (including a constant relative volatility model as was used by Billingham and Lockett and here).
Maldistribution is modelled by splitting the liquid flow so that the flow to the left differs from the flow to the right as shown on the flowsheet.
One small difference between our model and theirs is that they did not split the vapor flow as shown in Fig. 11; rather they assumed the gas flow to the base of each column to be the same.
We can obtain the same result by specifying a gas flow to the stream splitter that is twice that needed and a split ratio of 50 percent.
|
| [fsd] [png] |
 Extractive Distillation DMC/Methanol with o-Xylene (2/3/2025)
inspired by You et al. Separation and Purification Technology 350 (2024) pp. 127893 The Wilson parameters were fitted in ChemSep against VLE data from other sources as the values in the original source are incorrect.
Added heat integration by interchanging the feed with the hot solvent from the solvent recovery column. This vaporizes the feed to a large extent. The number of stages in the solvent recovery column were increased as to provide further savings.
The overal heat requirements reduced by more than a quarter.
Extractive Distillation DMC/Methanol with o-Xylene (2/3/2025)
inspired by You et al. Separation and Purification Technology 350 (2024) pp. 127893 The Wilson parameters were fitted in ChemSep against VLE data from other sources as the values in the original source are incorrect.
Added heat integration by interchanging the feed with the hot solvent from the solvent recovery column. This vaporizes the feed to a large extent. The number of stages in the solvent recovery column were increased as to provide further savings.
The overal heat requirements reduced by more than a quarter.
|
| [fsd] [png] |
 Snohvit LNG Cycle (2/2/2025)
Snohvit is an offshore natural gas reservoir located about 140 km north of Norway.
This simulation of the LNG liquefaction plant at Snohvit was inspired by the Masters thesis of Tormud Stugu (NTNU, 2003)
with modest modifications motivated by the Ph.D. thesis work of Anne Berit Ryan (NTNU, 2011) and the Hammerfest LNG Impact Assessment.
Links to all of these and other sources can be found on the flowsheet itself.
Simulated from data in from the Energy Plant Assessment report "Hammerfest LNG - Energianlegg Konsekvensutredning RA-SNO-00255" and 2003 MS thesis of Tormod Stugu at NTNU.
Snohvit LNG Cycle (2/2/2025)
Snohvit is an offshore natural gas reservoir located about 140 km north of Norway.
This simulation of the LNG liquefaction plant at Snohvit was inspired by the Masters thesis of Tormud Stugu (NTNU, 2003)
with modest modifications motivated by the Ph.D. thesis work of Anne Berit Ryan (NTNU, 2011) and the Hammerfest LNG Impact Assessment.
Links to all of these and other sources can be found on the flowsheet itself.
Simulated from data in from the Energy Plant Assessment report "Hammerfest LNG - Energianlegg Konsekvensutredning RA-SNO-00255" and 2003 MS thesis of Tormod Stugu at NTNU.
|
| [fsd] [png] [pdf] |
 Tall Oils (1/1/2025)
are the fatty acids obtained as a by-product of the Kraft wood pulp manufacturing process, see Tall Oil Production and Processing.
Originally, the Crude Tall Oil (CTO) was separated by means of steam distillation, however, this required a large amount of steam.
With the advent of structured packings in the early 1980s, dry distillation under deep vacuum became possible, where the relative volatilities between the components is larger.
The flowsheet uses the USA pine 'base' case as described in Chemical Engineering Research and Design 170 (2021) pp. 314-328.
First water is removed by drawing vacuum, then the CTO is split in four distillation steps into heavy pitch, pure rosin, resin acids, Tall Oil Fatty Acids (TOFA) as side draw) and Distilled Tall Oil (DTO) as bottoms educt.
Here the columns are equiped with circulating reflux sections acting as low pressure drop condensers as to keep all reboilers under 20 mbar to prevent product degradation.
Tall Oils (1/1/2025)
are the fatty acids obtained as a by-product of the Kraft wood pulp manufacturing process, see Tall Oil Production and Processing.
Originally, the Crude Tall Oil (CTO) was separated by means of steam distillation, however, this required a large amount of steam.
With the advent of structured packings in the early 1980s, dry distillation under deep vacuum became possible, where the relative volatilities between the components is larger.
The flowsheet uses the USA pine 'base' case as described in Chemical Engineering Research and Design 170 (2021) pp. 314-328.
First water is removed by drawing vacuum, then the CTO is split in four distillation steps into heavy pitch, pure rosin, resin acids, Tall Oil Fatty Acids (TOFA) as side draw) and Distilled Tall Oil (DTO) as bottoms educt.
Here the columns are equiped with circulating reflux sections acting as low pressure drop condensers as to keep all reboilers under 20 mbar to prevent product degradation.
|
| [fsd] [png] |
 VRC PP-Splitter (1/1/2025)
Propylene/Propane splitters have low relative volatilities and thus require high reflux ratios to obtain the required Propylene purity of 99.6 %.
As such, they lend themselves uniquely for heat pumping, lowering the operating pressure from around 20 to 12 bar. This provindes a cheaper column shell.
Further optimization can be done by preheating the feed with the cold reflux, reducing the partial reflash that otherwise increases the compressor power requirements unnecessarily
(see Christopher et al. Ind.Eng.Chem.Res. 56 p.14557 (2017)).
This feed/reflux exchanger also vaporizes the feed, which lowers the reboiler duty and leads to a uniform column diameter.
The correct Vapor Liquid Equilbrium (VLE) model for PP splitters is a critical issue: A deviation of 1 percent in the relative volatility may already create a 20-30 tray count difference.
In the past vendors used EoS without pure component corrections which led to use of all kinds of different Binary Interaction Parameters (BIP).
Use of Matthias-Copeman parameters allows for a more precise VLE description of the system with SRK and a constant BIP.
VRC PP-Splitter (1/1/2025)
Propylene/Propane splitters have low relative volatilities and thus require high reflux ratios to obtain the required Propylene purity of 99.6 %.
As such, they lend themselves uniquely for heat pumping, lowering the operating pressure from around 20 to 12 bar. This provindes a cheaper column shell.
Further optimization can be done by preheating the feed with the cold reflux, reducing the partial reflash that otherwise increases the compressor power requirements unnecessarily
(see Christopher et al. Ind.Eng.Chem.Res. 56 p.14557 (2017)).
This feed/reflux exchanger also vaporizes the feed, which lowers the reboiler duty and leads to a uniform column diameter.
The correct Vapor Liquid Equilbrium (VLE) model for PP splitters is a critical issue: A deviation of 1 percent in the relative volatility may already create a 20-30 tray count difference.
In the past vendors used EoS without pure component corrections which led to use of all kinds of different Binary Interaction Parameters (BIP).
Use of Matthias-Copeman parameters allows for a more precise VLE description of the system with SRK and a constant BIP.
|
| [fsd] [png] |
 Ammonolysis of Phenol to Aniline (25/11/2024)
process as shown in Figure 20.2 on page 366 of Synthetic Nitrogen Products by Gary R. Maxwell (Kluwer, 2004).
The flowsheet uses a simplified fixed conversion reactor for the reaction to main product Aniline and the side product DiPhenyl Amine (DPA).
The CS/COPP package uses the SRK-UMR to handle ammonia as a light gas while still being able to compute the nonideal interactions between Water and Aniline.
Ammonolysis of Phenol to Aniline (25/11/2024)
process as shown in Figure 20.2 on page 366 of Synthetic Nitrogen Products by Gary R. Maxwell (Kluwer, 2004).
The flowsheet uses a simplified fixed conversion reactor for the reaction to main product Aniline and the side product DiPhenyl Amine (DPA).
The CS/COPP package uses the SRK-UMR to handle ammonia as a light gas while still being able to compute the nonideal interactions between Water and Aniline.
|
| [fsd] [png] |
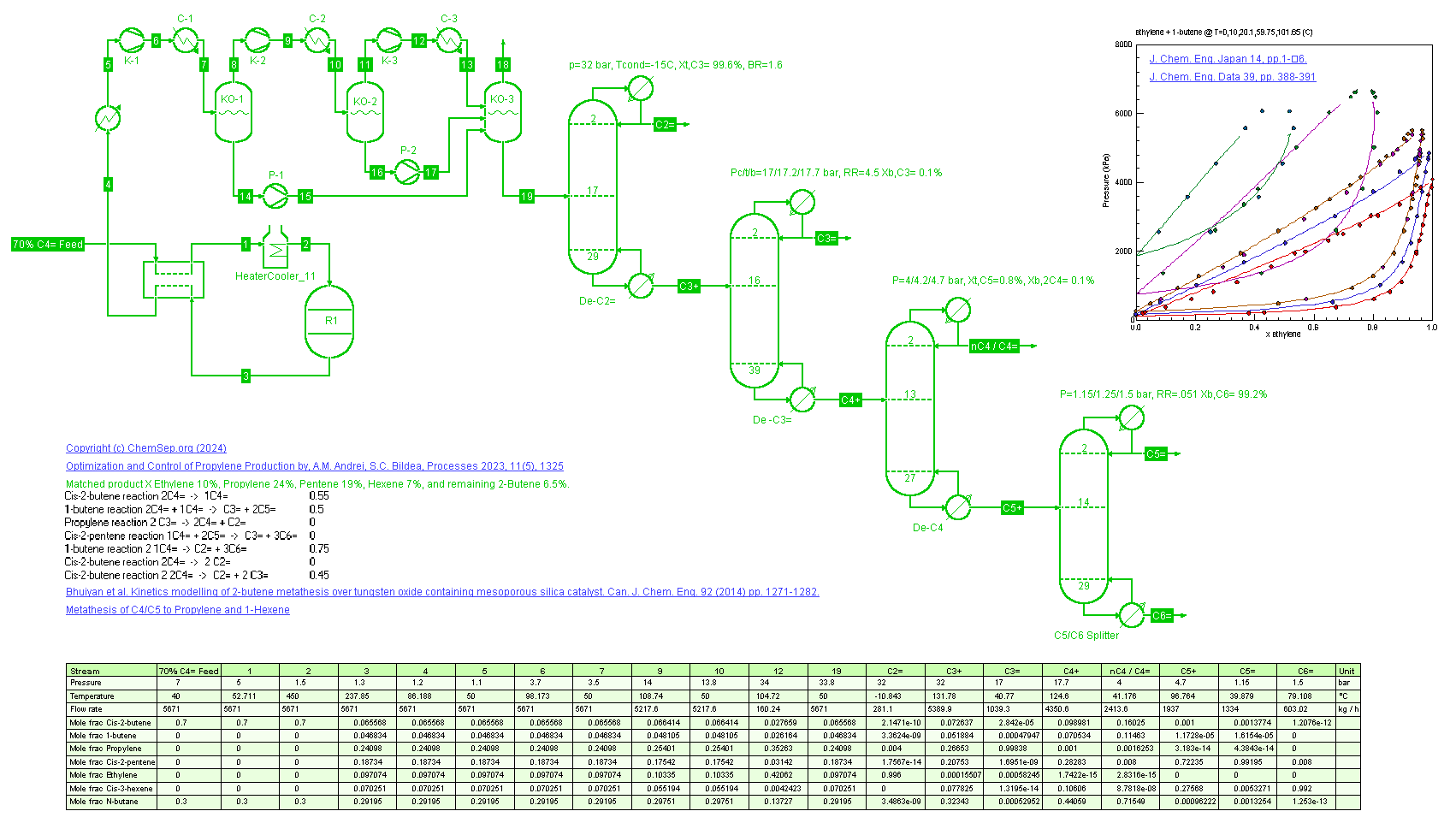 Butene Metathesis (22/01/2024)
as described in Optimization and Control of Propylene Production
by A. Andrei and S Bildea, Processes 2023, 11(5), p. 1325. Using a fixed conversion reactor the Butene metathesis to Propylene and 3-Hexane is simulated, providing a route to more Propylene for refinery feed.
Note that the 3-Hexene can be used to generate high RON alkylates or isomerized to produce 1-hexene, a valuable alpha-olefin.
The column pressures were optimized differently than described in the article. Except for the deethenizer, all condensers are designed to operate with cooling water and the process temperatures are set at 40 C.
The numbers of stages and Reflux Ratio were set as per FUG analysis using a 1.2 RR/RRmin and a 99% purity for the C5= and C6=.
The DeC3= column was sized to produce polymer grade Propylene of 99.8%. Similarly, the Ethyelne purity is 99.5% to produce polymer grade Ethylene.
On all purities we added a small margin for control and fluctuations.
Butene Metathesis (22/01/2024)
as described in Optimization and Control of Propylene Production
by A. Andrei and S Bildea, Processes 2023, 11(5), p. 1325. Using a fixed conversion reactor the Butene metathesis to Propylene and 3-Hexane is simulated, providing a route to more Propylene for refinery feed.
Note that the 3-Hexene can be used to generate high RON alkylates or isomerized to produce 1-hexene, a valuable alpha-olefin.
The column pressures were optimized differently than described in the article. Except for the deethenizer, all condensers are designed to operate with cooling water and the process temperatures are set at 40 C.
The numbers of stages and Reflux Ratio were set as per FUG analysis using a 1.2 RR/RRmin and a 99% purity for the C5= and C6=.
The DeC3= column was sized to produce polymer grade Propylene of 99.8%. Similarly, the Ethyelne purity is 99.5% to produce polymer grade Ethylene.
On all purities we added a small margin for control and fluctuations.
|
| [fsd] [png] |
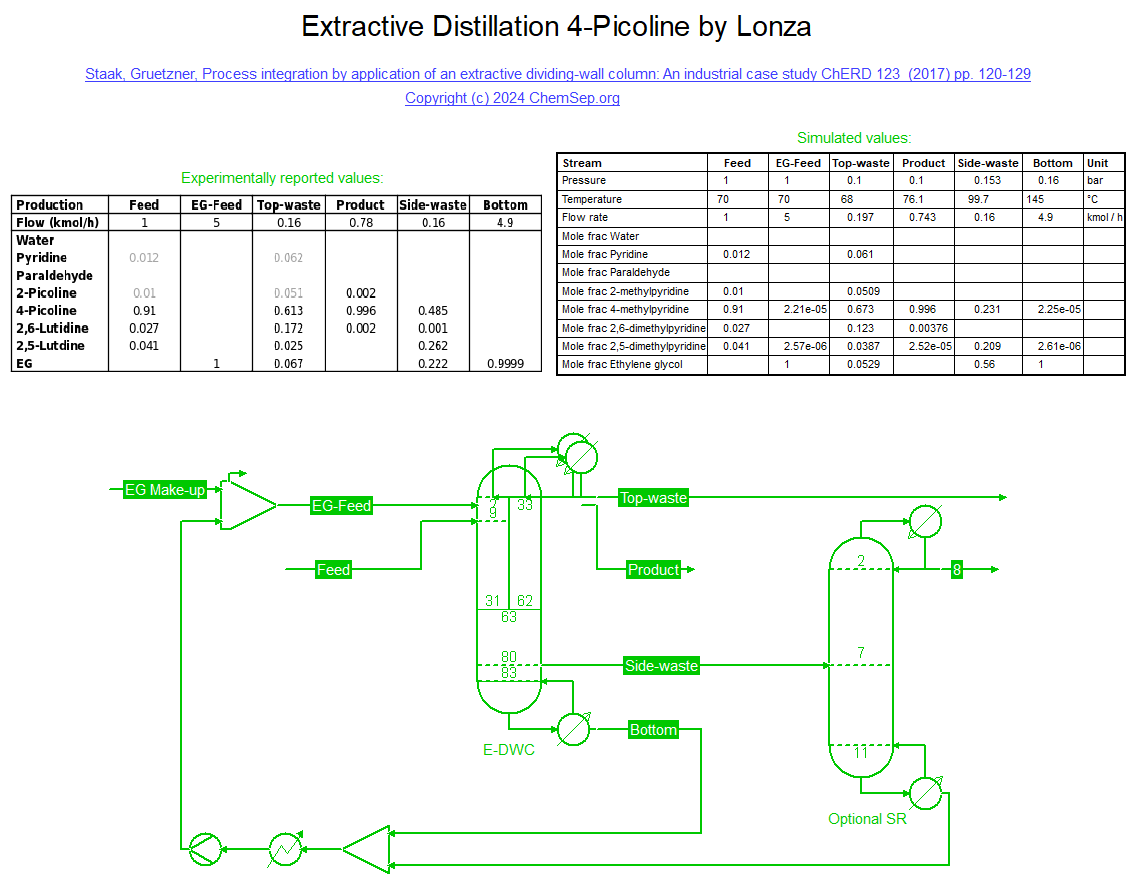 Lonza Extractive Distillation separation of 4-Picoline from side-product 2,6-Lutidine using a Top-Split Dividing Wall Columns (17/02/2024)
as described in "Staak and Gruetzner, Process integration by application of an extractive dividing-wall column: An industrial case study ChERD 123 (2017) pp. 120-129.
This is an extractive distillation of a close-boiling zeotropic ternary mixture using Ethylene Glycol as extractive agent. This can be done in a single column under vacuum conditions.
Lonza Extractive Distillation separation of 4-Picoline from side-product 2,6-Lutidine using a Top-Split Dividing Wall Columns (17/02/2024)
as described in "Staak and Gruetzner, Process integration by application of an extractive dividing-wall column: An industrial case study ChERD 123 (2017) pp. 120-129.
This is an extractive distillation of a close-boiling zeotropic ternary mixture using Ethylene Glycol as extractive agent. This can be done in a single column under vacuum conditions.
|
| [fsd] [png] |
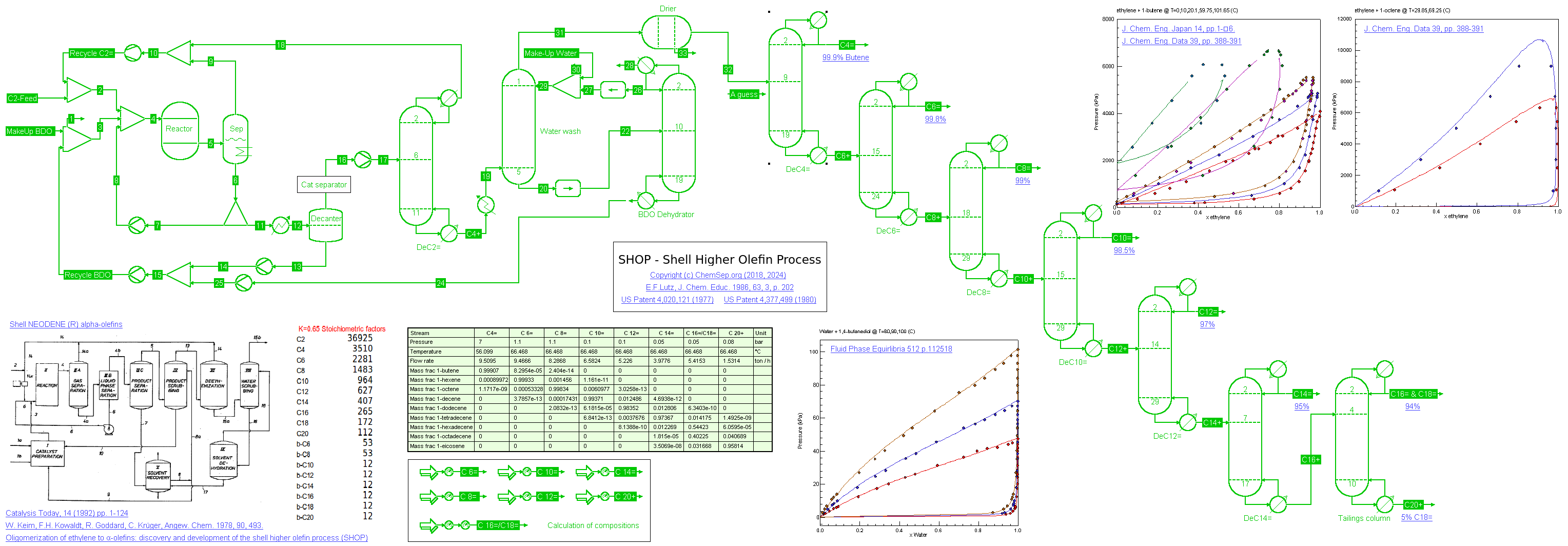 Shell Higher Olefin Process (06/06/2018 and 25/02/2024)
The Shell Higher Olefin Process (SHOP) as described in US Patent 4,020,121 (1977) and
Catalysis Today Vol. 14, 1992, p.1. See also E.F.Lutz, J. Chem. Educ. 1986, 63, 3, p. 202.
A stream of 50 t/h Ethylene is oligomerized under 90 C and 90 bar pressure in 1,4-Butanediol (BDO) as solvent in a solvent to oligimer ratio of 10:1 producing about 76 KTA 1-butene and 324 KTA higher linear alpha-olefins.
We use a fixed conversion reactor with a 80% Ethylene conversion and a stoichiometry that represents a K=Cn+2/Cn=0.65 to represent the complex three-phase reators.
The oligomer phase is distilled to recycle the unreacted Ethylene. After a water-wash to remove the remaining solvent, the oligomers are distilled in a direct sequence.
Purity of the final products are as per Shell NEODENE (R) product specifications.
Shell Higher Olefin Process (06/06/2018 and 25/02/2024)
The Shell Higher Olefin Process (SHOP) as described in US Patent 4,020,121 (1977) and
Catalysis Today Vol. 14, 1992, p.1. See also E.F.Lutz, J. Chem. Educ. 1986, 63, 3, p. 202.
A stream of 50 t/h Ethylene is oligomerized under 90 C and 90 bar pressure in 1,4-Butanediol (BDO) as solvent in a solvent to oligimer ratio of 10:1 producing about 76 KTA 1-butene and 324 KTA higher linear alpha-olefins.
We use a fixed conversion reactor with a 80% Ethylene conversion and a stoichiometry that represents a K=Cn+2/Cn=0.65 to represent the complex three-phase reators.
The oligomer phase is distilled to recycle the unreacted Ethylene. After a water-wash to remove the remaining solvent, the oligomers are distilled in a direct sequence.
Purity of the final products are as per Shell NEODENE (R) product specifications.
|
| [fsd] [png] |
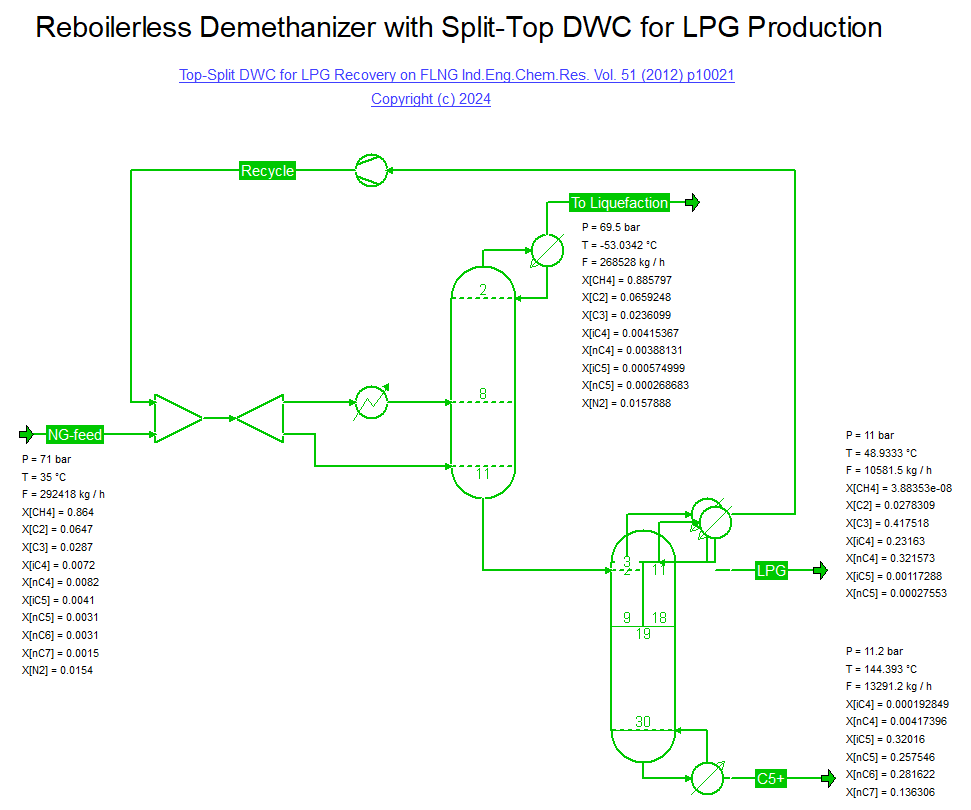 Lee et al. published a Top-Split DWC for LPG Recovery on a FLNG facility (28/02/2024) in Ind.Eng.Chem.Res. Vol. 51 (2012) p. 10021.
The bottoms of a demethanizer is fed to a top-split DWC where the rich Methane/Ethane recycle is taken off first (left of the dividing wall),
whereas a LPG with 97% C3/C4 content is produced on the right hand side of the wall. The column bottoms is a stabilized condensate with approximately 0.05% C4 content.
Note that the demethanizer column is run with a split feed where warm feed is used as boilup vapor to avoid the need for a reboiler.
Lee et al. published a Top-Split DWC for LPG Recovery on a FLNG facility (28/02/2024) in Ind.Eng.Chem.Res. Vol. 51 (2012) p. 10021.
The bottoms of a demethanizer is fed to a top-split DWC where the rich Methane/Ethane recycle is taken off first (left of the dividing wall),
whereas a LPG with 97% C3/C4 content is produced on the right hand side of the wall. The column bottoms is a stabilized condensate with approximately 0.05% C4 content.
Note that the demethanizer column is run with a split feed where warm feed is used as boilup vapor to avoid the need for a reboiler.
|
| [fsd] [png] |
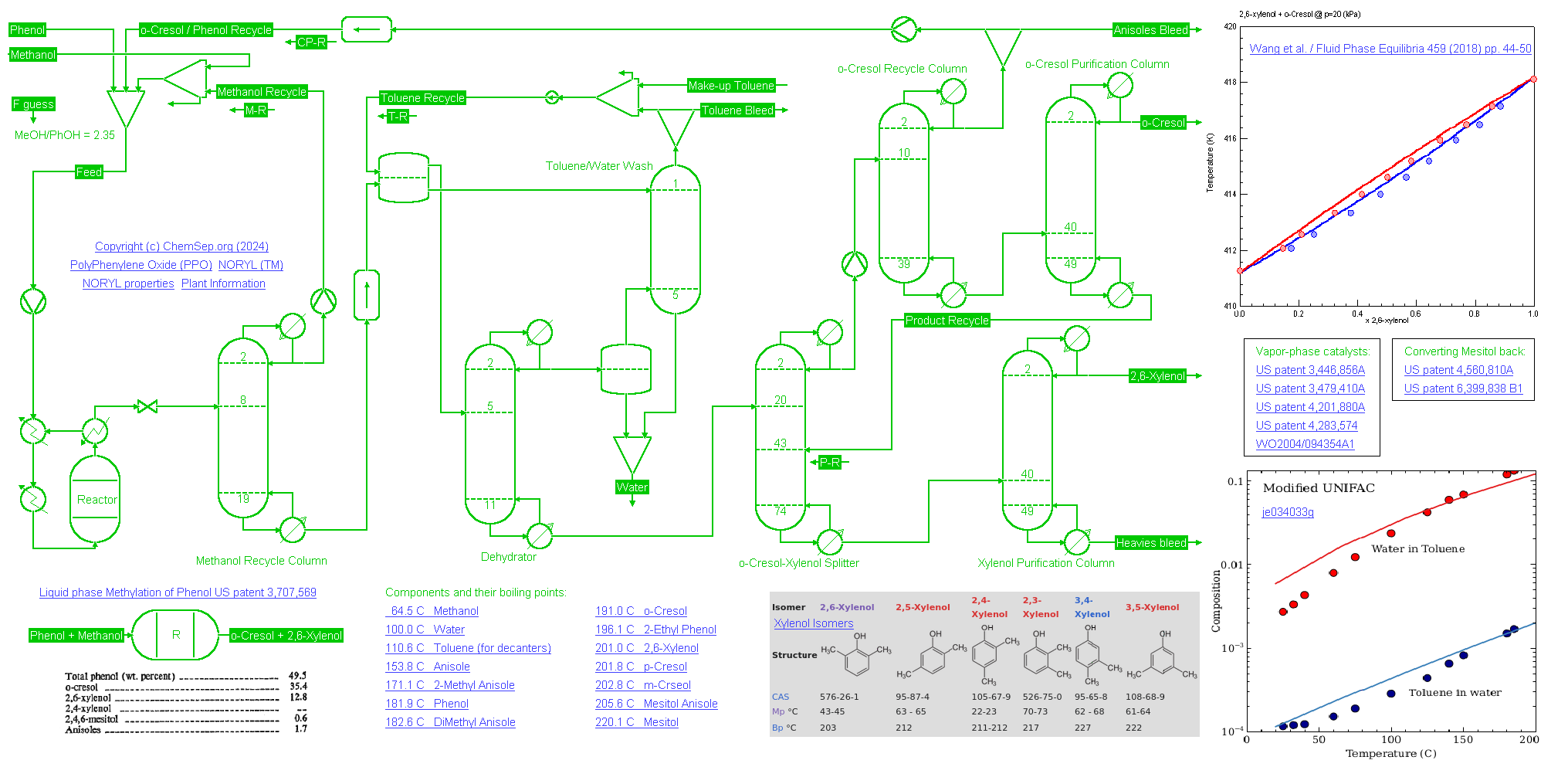 45 KTA 26-Xylenol or (Di-Methyl Phenol) process plant (02/02/2024) using liquid phase methylation of Phenol US patent 3,707,569
Phenol is selectively methylated at the ortho-position by reacting it in a 2.3 to 1 ratio with Methanol in the liquid phase at 250 deg C and 150 bar over a catalyst.
Single-pass conversion reached 50 percent with a selectivity of 70 percent to o-Cresol and 25 percent to 2,6-Xylenol. The unreacted Methanol is recycled to increase yield.
Phenol in the produced water is removed using a Toluene liquid-liquid extraction. Unreacted Phenol and a part of the produced o-Cresol is recycled.
The disadvantage in this process is that the catalyst produces relatively large amounts of Anisoles that form a close boiling systems with Phenol and other species. As such, a bleed must be taken.
45 KTA 26-Xylenol or (Di-Methyl Phenol) process plant (02/02/2024) using liquid phase methylation of Phenol US patent 3,707,569
Phenol is selectively methylated at the ortho-position by reacting it in a 2.3 to 1 ratio with Methanol in the liquid phase at 250 deg C and 150 bar over a catalyst.
Single-pass conversion reached 50 percent with a selectivity of 70 percent to o-Cresol and 25 percent to 2,6-Xylenol. The unreacted Methanol is recycled to increase yield.
Phenol in the produced water is removed using a Toluene liquid-liquid extraction. Unreacted Phenol and a part of the produced o-Cresol is recycled.
The disadvantage in this process is that the catalyst produces relatively large amounts of Anisoles that form a close boiling systems with Phenol and other species. As such, a bleed must be taken.
|
| [fsd] [png] |
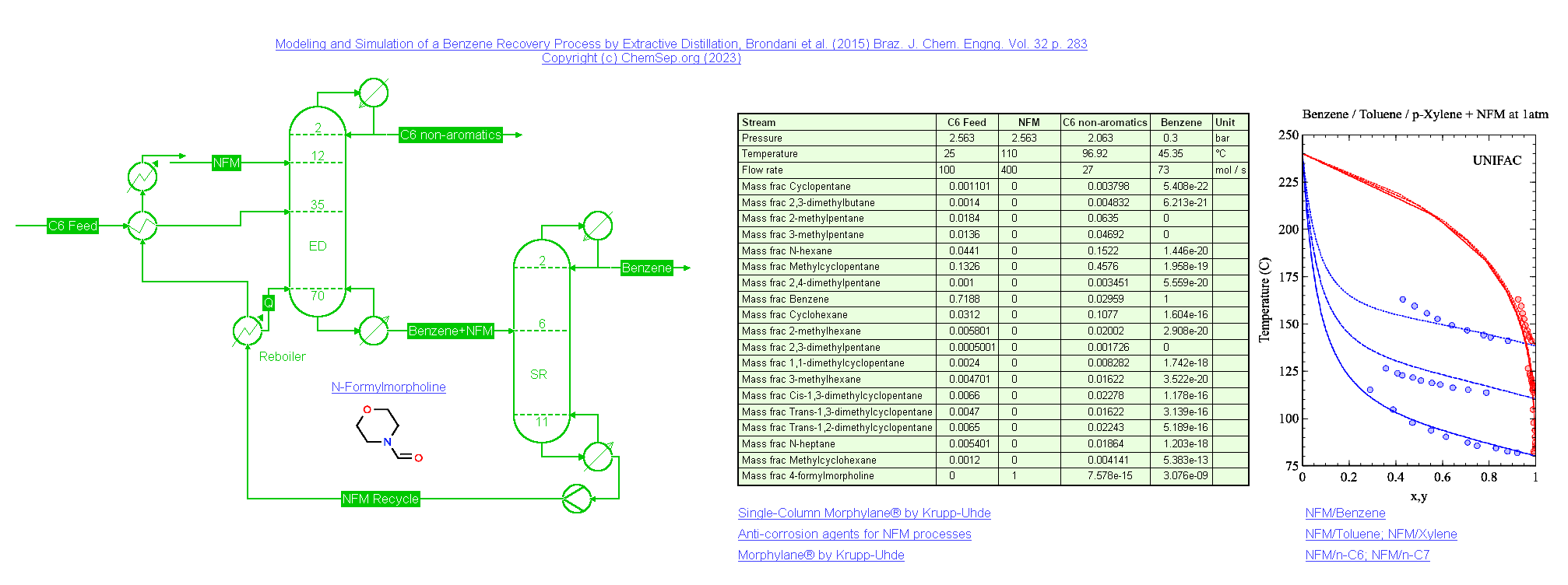 Extractive Distillation of Benzene using NFM (25/12/2023)
Extractive distillation of Benzene using N-Formyl Morpholine (NFM) based on Brondani et al. (2015) Braz. J. Chem. Engng. Vol. 32 p. 283.
Benzene and NFM are separated from a mixture of C6 non-aromatic compounds in an Extractive Distillation (ED) column. Benzene is recovered from the mixture
with NFM under vacuum conditions in the Solvent Recovery (SR) column, which is heat integrated with the reboiler of the ED column, resulting in a significant
reduction of energy required. The recovered NFM from the SR column is recycled back into the ED column. This process is simulated using the UNIFAC activity
coefficient model, with modified interaction parameters for the NFM-ACH and NFM-ACCH2 groups on the hand of Vapor-Liquid Equilibria data of NFM with Benzene,
Toluene, and p-Xylene.
Extractive Distillation of Benzene using NFM (25/12/2023)
Extractive distillation of Benzene using N-Formyl Morpholine (NFM) based on Brondani et al. (2015) Braz. J. Chem. Engng. Vol. 32 p. 283.
Benzene and NFM are separated from a mixture of C6 non-aromatic compounds in an Extractive Distillation (ED) column. Benzene is recovered from the mixture
with NFM under vacuum conditions in the Solvent Recovery (SR) column, which is heat integrated with the reboiler of the ED column, resulting in a significant
reduction of energy required. The recovered NFM from the SR column is recycled back into the ED column. This process is simulated using the UNIFAC activity
coefficient model, with modified interaction parameters for the NFM-ACH and NFM-ACCH2 groups on the hand of Vapor-Liquid Equilibria data of NFM with Benzene,
Toluene, and p-Xylene.
|
| [fsd] [png] |
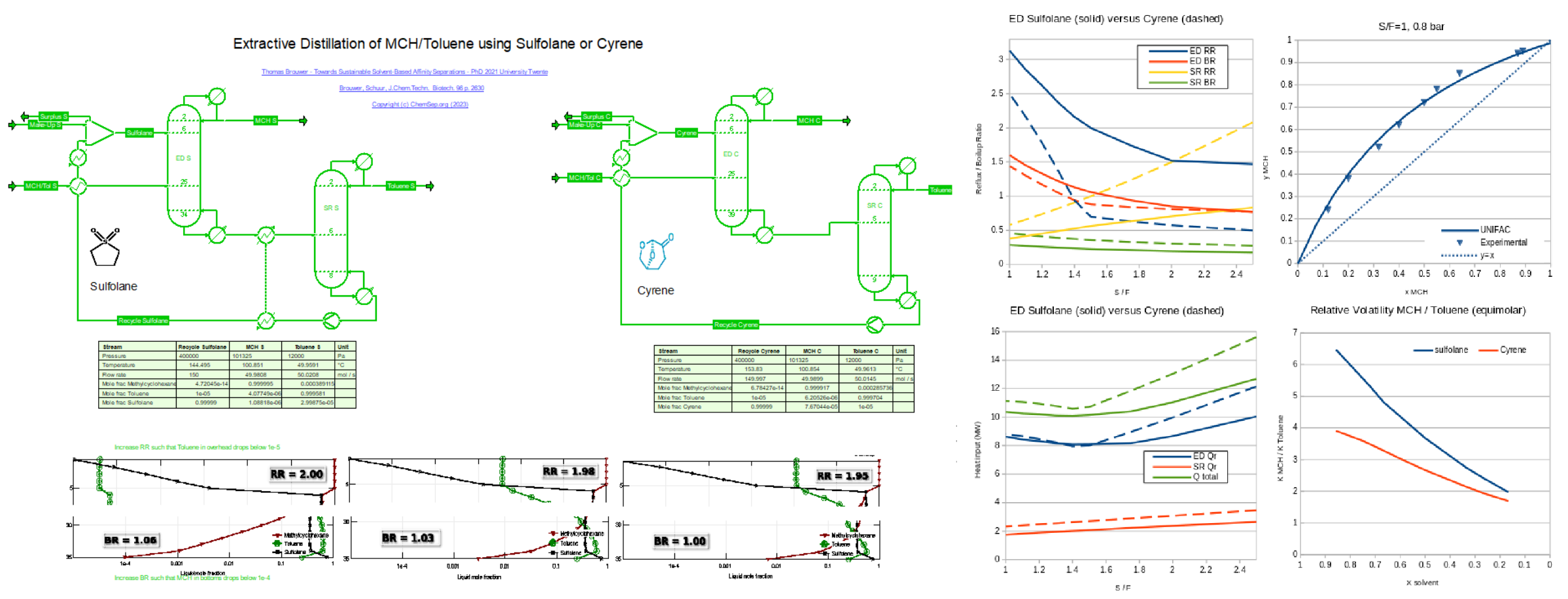 Extraction Distillation of Aromatics with Sulfolane and bio-based solvent Cyrene (31/10/2023)
from a stabilized refinery reformate after figures 10.2 and 10.6 from T.Brouwer (PhD TU Twente 2021).
See alse Brouwer, Schuur, J.Chem.Techn. & Biotech. 96 p. 2630.
Here the aromatics are represented by Toluene, and the naphthenic compoinents by MethylCycloHexane (MCH). The separation is simulated using the UNIFAC activity coefficient model, which allows inclusion of any hydrocarbon compounds.
The aromatics are recovered from the solvents under vacuum conditions in the Solvent Recovery (SR) column, which is heat integrated with the feed to the ED column.
For Sulfolane, the SR bottoms is hot enough such that an additional heat integration step can be done. This step lowers the overall energy consumption for Sulfolane compared to Cyrene.
A parametric study was performed for different Solvent/Feed ratios to determine the optimum. For Sulfolane this is at S/F=1.5.
Extraction Distillation of Aromatics with Sulfolane and bio-based solvent Cyrene (31/10/2023)
from a stabilized refinery reformate after figures 10.2 and 10.6 from T.Brouwer (PhD TU Twente 2021).
See alse Brouwer, Schuur, J.Chem.Techn. & Biotech. 96 p. 2630.
Here the aromatics are represented by Toluene, and the naphthenic compoinents by MethylCycloHexane (MCH). The separation is simulated using the UNIFAC activity coefficient model, which allows inclusion of any hydrocarbon compounds.
The aromatics are recovered from the solvents under vacuum conditions in the Solvent Recovery (SR) column, which is heat integrated with the feed to the ED column.
For Sulfolane, the SR bottoms is hot enough such that an additional heat integration step can be done. This step lowers the overall energy consumption for Sulfolane compared to Cyrene.
A parametric study was performed for different Solvent/Feed ratios to determine the optimum. For Sulfolane this is at S/F=1.5.
|
| [fsd] [png] |
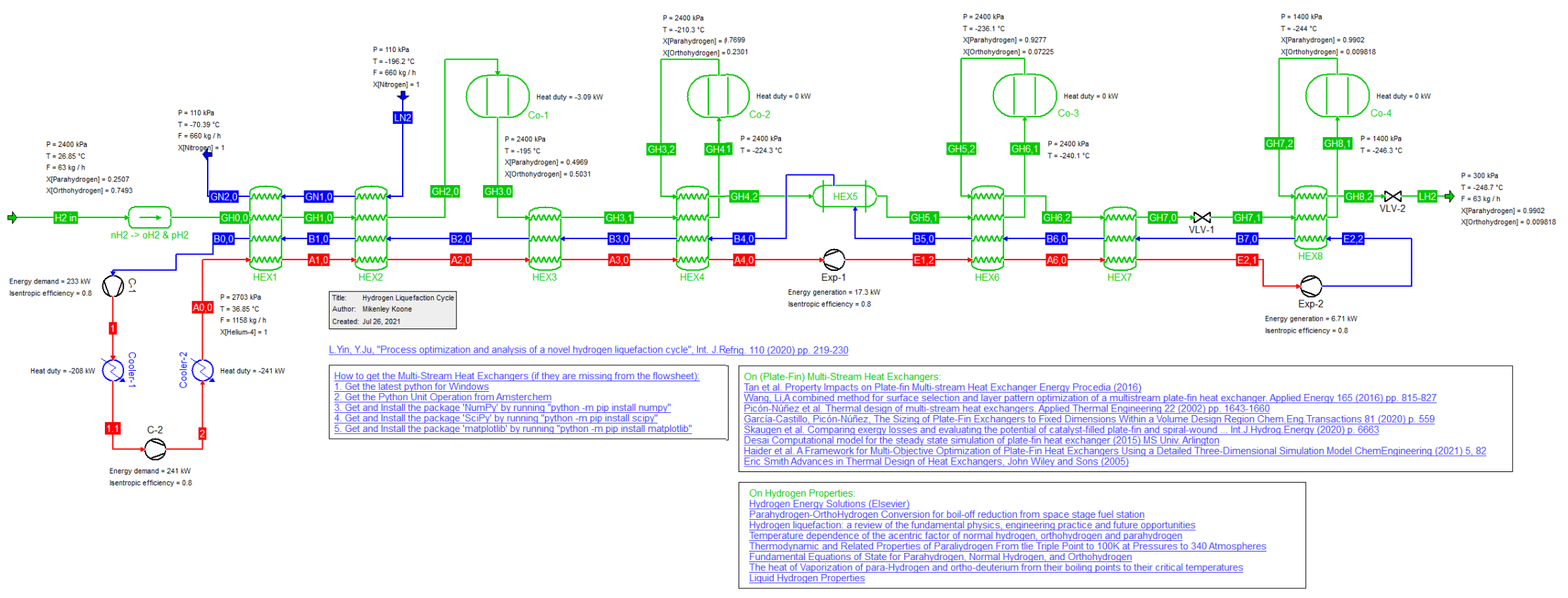 Novel Hydrogren Liquefaction Cycle (26/3/2023)
Novel hydrogen Liquefaction cycle as described by L. Yin and Y. Ju, Int. J.Refrig. 110 (2020) pp. 219-230, which utilizes a liquid nitrogen pre-cooling system as well as a helium cryogenic cycle.
The regular Hydrogen component is normally simulated as a single molecule but in this cycle we distinguish between the ortho- and para-magnetic isomers Hydrogen actually consists of.
Hence we first convert a stream consisting of regular Hydrogen component into the two isomers. At room temperature Hydrogen consists of 75 percent ortho-H2 and 25 percent para-H2.
The conversion between the two isomers para-hydrogen and ortho-hydrogen is achieved in a series of reactors loaded with Iron (III) Hydroxide (Fe(OH)3) as the catalyst.
The original publication did not account for the energy difference between ortho- and para-Hydrogen. This was corrected by inclusion of an offset for the Heat of Formation of ortho-H2.
This is why the conversion of regular Hydrogen into its two isomers has a heat effect that in reality isn't there.
Novel Hydrogren Liquefaction Cycle (26/3/2023)
Novel hydrogen Liquefaction cycle as described by L. Yin and Y. Ju, Int. J.Refrig. 110 (2020) pp. 219-230, which utilizes a liquid nitrogen pre-cooling system as well as a helium cryogenic cycle.
The regular Hydrogen component is normally simulated as a single molecule but in this cycle we distinguish between the ortho- and para-magnetic isomers Hydrogen actually consists of.
Hence we first convert a stream consisting of regular Hydrogen component into the two isomers. At room temperature Hydrogen consists of 75 percent ortho-H2 and 25 percent para-H2.
The conversion between the two isomers para-hydrogen and ortho-hydrogen is achieved in a series of reactors loaded with Iron (III) Hydroxide (Fe(OH)3) as the catalyst.
The original publication did not account for the energy difference between ortho- and para-Hydrogen. This was corrected by inclusion of an offset for the Heat of Formation of ortho-H2.
This is why the conversion of regular Hydrogen into its two isomers has a heat effect that in reality isn't there.
|
| [fsd] [png] |
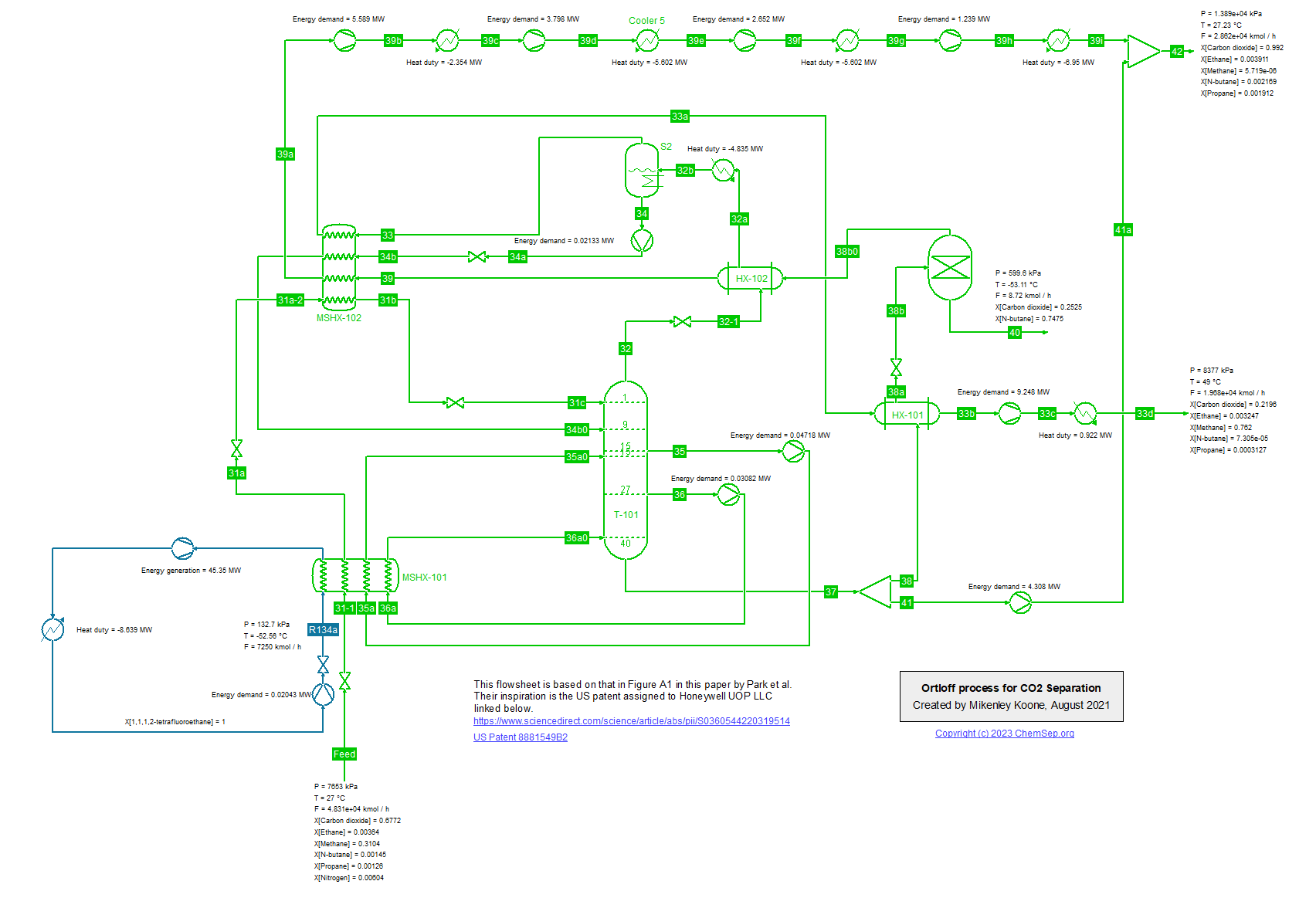 Energy efficient process for CO2 removal from Natural Gas for CCS (14/2/2023)
Park et al. (2021) (Energy, Vol 214, p. 118844) describe an energy efficient process for low temperature CO2 removal from natural gas using an R134a refrigeration cycle.
This patented process, like the SPREX process, is limited to produce a methane-rich product with 20-25 mol% of CO2 concentration. To produce natural gas with sales specification, an additional CO2 removal unit will be required.
The simulation here uses the Peng-Robinson Equation of State.
Energy efficient process for CO2 removal from Natural Gas for CCS (14/2/2023)
Park et al. (2021) (Energy, Vol 214, p. 118844) describe an energy efficient process for low temperature CO2 removal from natural gas using an R134a refrigeration cycle.
This patented process, like the SPREX process, is limited to produce a methane-rich product with 20-25 mol% of CO2 concentration. To produce natural gas with sales specification, an additional CO2 removal unit will be required.
The simulation here uses the Peng-Robinson Equation of State.
|
| [fsd] [png] |
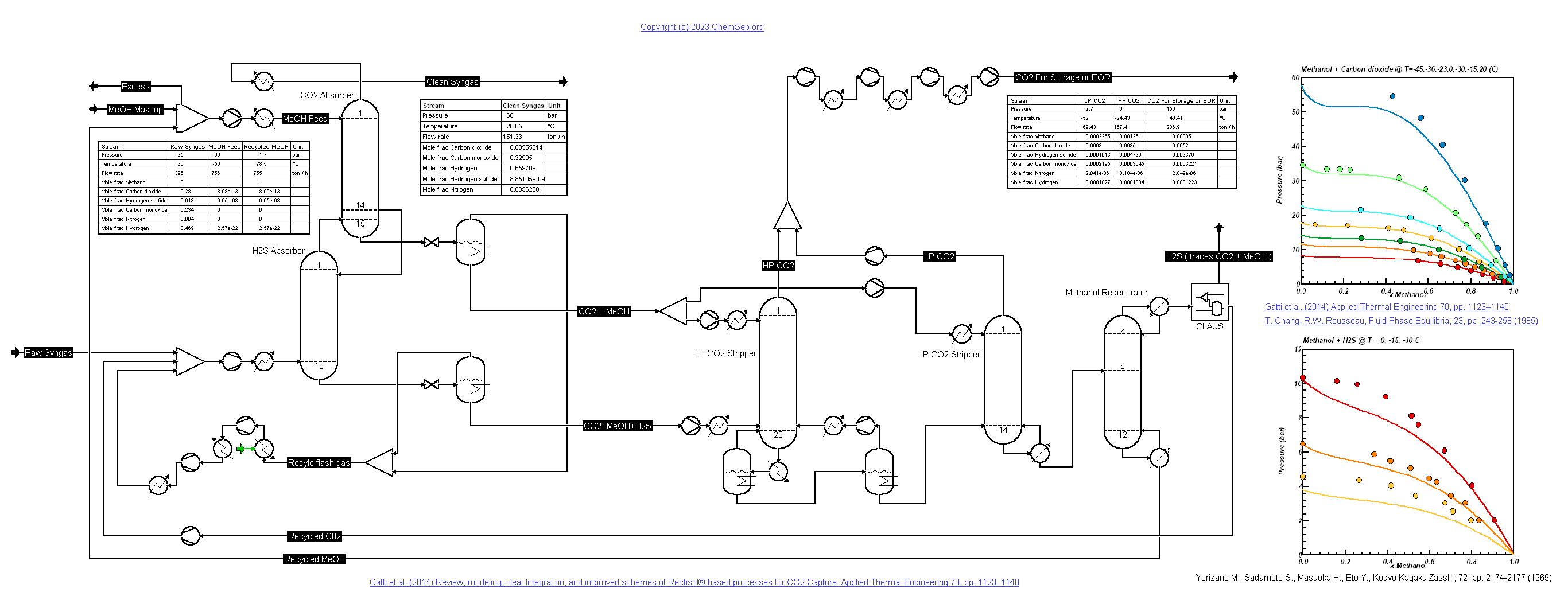 Rectisol Process for SynGas with CO2 for EOR (15/1/2023)
This process describes the Rectisol process to remove all CO2 from syn-gas by absorbing in cold methanol (Rectisol process) as described by
Gatti et al. (2014).
Note that some processes may benefit from not removing all CO2 from the syngas. The CO2 is compressed to a pressure of 150 bar for reservoir
injection for Enhanced Oil Recovery purposes. The H2S removal is simplified and simulated as a simple Claus separation block.
Rectisol Process for SynGas with CO2 for EOR (15/1/2023)
This process describes the Rectisol process to remove all CO2 from syn-gas by absorbing in cold methanol (Rectisol process) as described by
Gatti et al. (2014).
Note that some processes may benefit from not removing all CO2 from the syngas. The CO2 is compressed to a pressure of 150 bar for reservoir
injection for Enhanced Oil Recovery purposes. The H2S removal is simplified and simulated as a simple Claus separation block.
|
| [fsd] [png] |
 Extraction of Aromatics with Sulfolane (16/7/2022)
from a refinery catalytic reformer stream after
Figure 10.1 in T.Brouwer (PhD TU Twente 2021). Aromatics are removed from the stabilized reformate using a liquid-liquid extraction column with Sulfolane.
The extractor is operated with a recycle of light C5 alkanes and Sulfolane is washed out from the raffinate with water.
The aromatics are recovered from the sulfolane under vacuum conditions.
Note that the LL extractor can be solved with internal LLE-thermodynamics or via a LLE Cape-Open Property Package (COPP).
For the latter we need to convert the specific streams from the VLE to the LLE COPP and vice-versa.
To visualize this we used different colors for the VLE streams (in blue) and LLE streams (in teal).
Extraction of Aromatics with Sulfolane (16/7/2022)
from a refinery catalytic reformer stream after
Figure 10.1 in T.Brouwer (PhD TU Twente 2021). Aromatics are removed from the stabilized reformate using a liquid-liquid extraction column with Sulfolane.
The extractor is operated with a recycle of light C5 alkanes and Sulfolane is washed out from the raffinate with water.
The aromatics are recovered from the sulfolane under vacuum conditions.
Note that the LL extractor can be solved with internal LLE-thermodynamics or via a LLE Cape-Open Property Package (COPP).
For the latter we need to convert the specific streams from the VLE to the LLE COPP and vice-versa.
To visualize this we used different colors for the VLE streams (in blue) and LLE streams (in teal).
|
| [fsd] [png] |
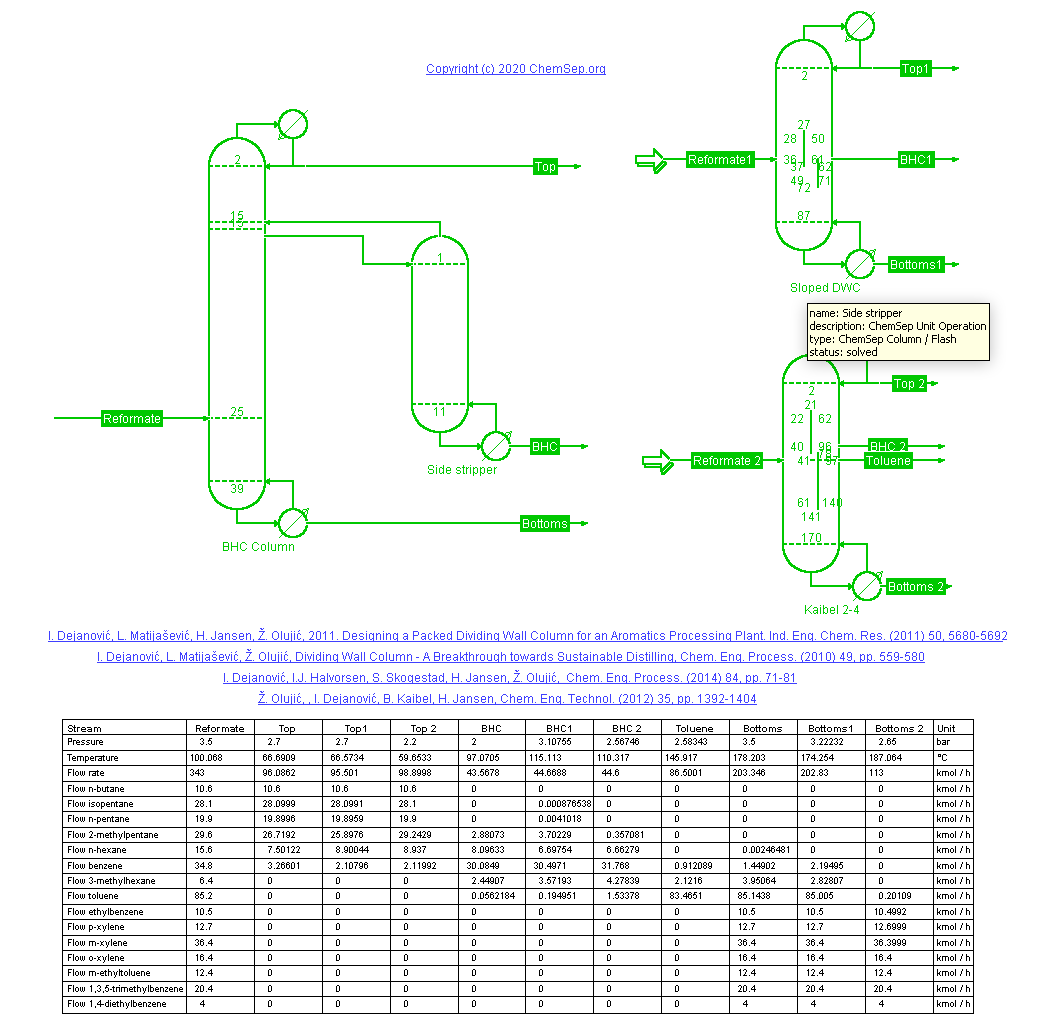 Reformate Splitter (1/7/2022) of a refinery catalytic reformer using a Dividing Wall Column (DWC) after
I. Dejanovic, L. Matijasevic, H. Jansen, Z. Olujic "Designing a Packed Dividing Wall Column for an Aromatics Processing Plant" Ind. Eng. Chem. Res. 50, pp. 5680-5692 (2011).
Benzene is removed from the Mogas pool in this reformate splitter. Traditionally this is done with a side-stripper
configuration producing a Benzene-rich heart-cut stream. This configuration is compared to a DWC with a sloped wall
and to the Kaibel column that adds a Toluene-rich product. By performing multiple separations in a single shell the
DWC requires less energy, less capital and less space than conventional columns-in-series and/or in-parallel configurations.
As such DWC are an important tool for the petro- and petrochemicals process industry towards a more sustainable way of
distilling products.
Reformate Splitter (1/7/2022) of a refinery catalytic reformer using a Dividing Wall Column (DWC) after
I. Dejanovic, L. Matijasevic, H. Jansen, Z. Olujic "Designing a Packed Dividing Wall Column for an Aromatics Processing Plant" Ind. Eng. Chem. Res. 50, pp. 5680-5692 (2011).
Benzene is removed from the Mogas pool in this reformate splitter. Traditionally this is done with a side-stripper
configuration producing a Benzene-rich heart-cut stream. This configuration is compared to a DWC with a sloped wall
and to the Kaibel column that adds a Toluene-rich product. By performing multiple separations in a single shell the
DWC requires less energy, less capital and less space than conventional columns-in-series and/or in-parallel configurations.
As such DWC are an important tool for the petro- and petrochemicals process industry towards a more sustainable way of
distilling products.
|
| [fsd] [png] |
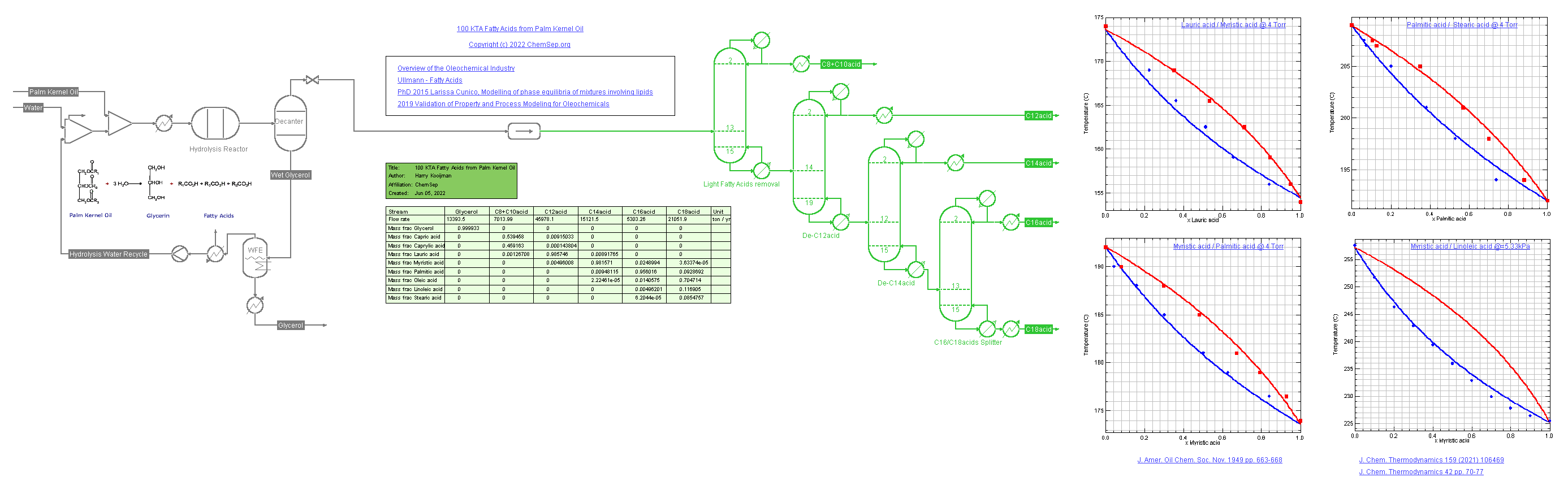 Process for Fatty Acids (1/6/2022) from Palm Kernel Oil adapted from a
Oleochemicals Chemicals 2011 presentation by OXITENO on their Brazilian process plants.
This flowsheet demonstrates the use of multiple K-models.
The SRK-UMR equation of state is used for the hydrolysis of the oil into fatty acids and the separation in C12, C14, C16, and C18 acids. Note that the C18 fatty acids are not lumped but split out over three fractions of Lineic, Linoleic, and Stearic acids.
Consequently, the DECHEMA-UNIFAC model is used for the separation train of the fatty alcohols in vacuum columns. Some of the binary Vapor-Liquid diagrams of the fatty acids are displayed on the flowsheet.
We use different colors to illustrate where each package is used: The units and streams using DECHEMA-UNIFAC are shown in green when solved.
Process for Fatty Acids (1/6/2022) from Palm Kernel Oil adapted from a
Oleochemicals Chemicals 2011 presentation by OXITENO on their Brazilian process plants.
This flowsheet demonstrates the use of multiple K-models.
The SRK-UMR equation of state is used for the hydrolysis of the oil into fatty acids and the separation in C12, C14, C16, and C18 acids. Note that the C18 fatty acids are not lumped but split out over three fractions of Lineic, Linoleic, and Stearic acids.
Consequently, the DECHEMA-UNIFAC model is used for the separation train of the fatty alcohols in vacuum columns. Some of the binary Vapor-Liquid diagrams of the fatty acids are displayed on the flowsheet.
We use different colors to illustrate where each package is used: The units and streams using DECHEMA-UNIFAC are shown in green when solved.
|
| [fsd] [png] |
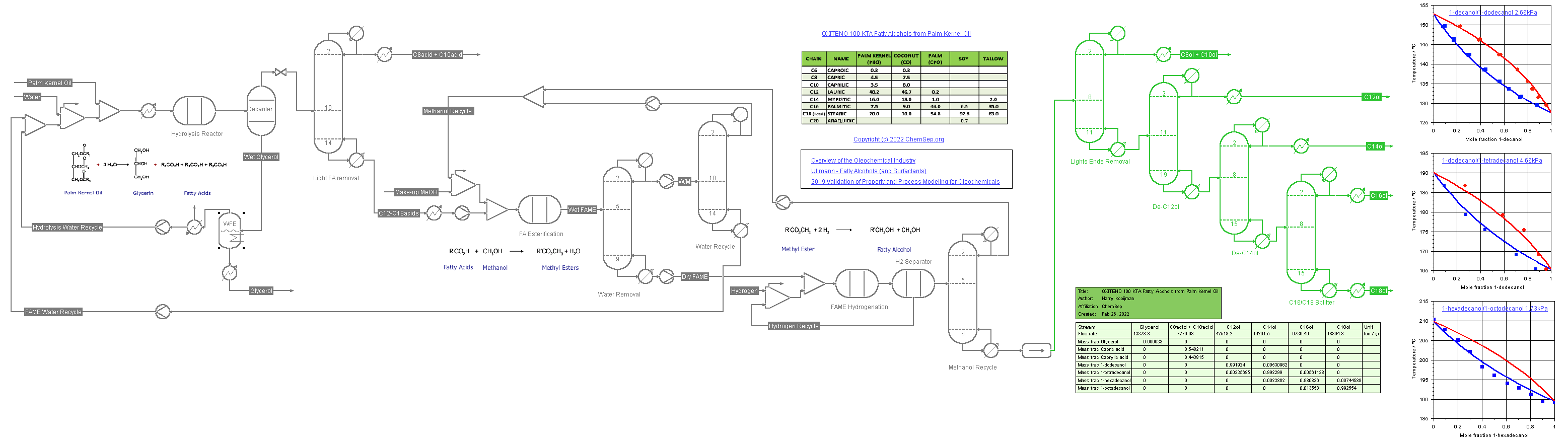 Process for Fatty Alcohols (1/6/2022) from Palm Kernel Oil adapted from a
Oleochemicals Chemicals 2011 presentation by OXITENO on their Brazilian process plants.
This flowsheet demonstrates the use of multiple K-models.
The SRK-UMR equation of state is used for the hydrolysis of the oil into fatty acids, the conversion of these to Fatty Acids Methyl Esters (FAME) and the hydrogenation of FAME to the fatty alcohols.
Consequently, the DECHEMA-UNIFAC model is used for the separation train of the fatty alcohols in vacuum columns. The binary Vapor-Liquid diagrams of the fatty acids are displayed on the flowsheet.
Note we also use different color schemes to illustrate where each package is used.
The units and streams using SRK-UMR display in dark grey when solved whereas those units and streams using DECHEMA-UNIFAC are shown in green when solved.
Process for Fatty Alcohols (1/6/2022) from Palm Kernel Oil adapted from a
Oleochemicals Chemicals 2011 presentation by OXITENO on their Brazilian process plants.
This flowsheet demonstrates the use of multiple K-models.
The SRK-UMR equation of state is used for the hydrolysis of the oil into fatty acids, the conversion of these to Fatty Acids Methyl Esters (FAME) and the hydrogenation of FAME to the fatty alcohols.
Consequently, the DECHEMA-UNIFAC model is used for the separation train of the fatty alcohols in vacuum columns. The binary Vapor-Liquid diagrams of the fatty acids are displayed on the flowsheet.
Note we also use different color schemes to illustrate where each package is used.
The units and streams using SRK-UMR display in dark grey when solved whereas those units and streams using DECHEMA-UNIFAC are shown in green when solved.
|
| [fsd] [png] |
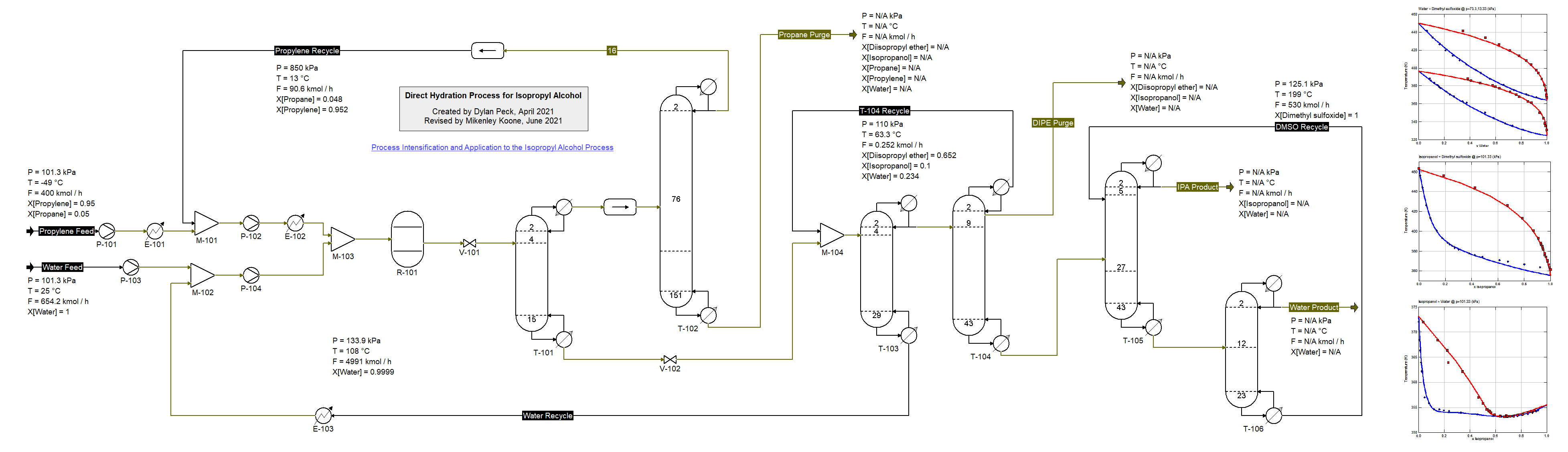 Process for Isopropyl Alcohol (25/7/2021) synthesis from Propylene and Water adapted from
Niu et al., Ind. Eng. Chem. Res. 2016, 55, 12, pp. 3614-3629. This flowsheet demonstrates the use of multiple K-models. The Peng-Robinson equation of state is used for the separation of propylene from propane allowing for the reactant to be recycle back to the reactor. The NRTL model is used for the remaining operations of the process and the binary interaction parameters have been fit to available experimental data for the nonideal binary mixtures involved in this process. Due to the possibility of three phases in the pairs containing Propane/Proylene and Water the UNIFAC model is used to predict those parameters. To increase temperature and prevent two liquid phases from forming, the first column utilizes a partial condenser. The most difficult separation in this process is between Water and Isopropyl Alcohol therefore the solvent DMSO (Dimethyl Sulfoxide) is introduced to the final separation section effectively allowing for a desirable final product of IPA. Niu et al. describe additional improvements that can made to this process.
Process for Isopropyl Alcohol (25/7/2021) synthesis from Propylene and Water adapted from
Niu et al., Ind. Eng. Chem. Res. 2016, 55, 12, pp. 3614-3629. This flowsheet demonstrates the use of multiple K-models. The Peng-Robinson equation of state is used for the separation of propylene from propane allowing for the reactant to be recycle back to the reactor. The NRTL model is used for the remaining operations of the process and the binary interaction parameters have been fit to available experimental data for the nonideal binary mixtures involved in this process. Due to the possibility of three phases in the pairs containing Propane/Proylene and Water the UNIFAC model is used to predict those parameters. To increase temperature and prevent two liquid phases from forming, the first column utilizes a partial condenser. The most difficult separation in this process is between Water and Isopropyl Alcohol therefore the solvent DMSO (Dimethyl Sulfoxide) is introduced to the final separation section effectively allowing for a desirable final product of IPA. Niu et al. describe additional improvements that can made to this process.
|
| [fsd] [png] |
 Ethanol-Amines process (15/7/2021).
Process for a SRI 45 KTA Mono-Ethanol-Amine (MEA) / Di-Ethanol-Amine (DEA) / Tri-Ethanol-Amine (TEA) production as per US 4,355,181
and described in Korean J. Chem. Eng Vol. 26, No. 6 pp. 1504-1511 (2009), using fixed conversion reactors and a substitute for the heavier ethanol amines. Two different ChemSep Cape-Open
Property Packages are used: The SRK-UMR is used to describe the VLE in the high pressure reactor section and the gamma-phi method with NRTL parameters predicted from UNIFAC is used for
the vacuum distillation of the ethanolamines.
Ethanol-Amines process (15/7/2021).
Process for a SRI 45 KTA Mono-Ethanol-Amine (MEA) / Di-Ethanol-Amine (DEA) / Tri-Ethanol-Amine (TEA) production as per US 4,355,181
and described in Korean J. Chem. Eng Vol. 26, No. 6 pp. 1504-1511 (2009), using fixed conversion reactors and a substitute for the heavier ethanol amines. Two different ChemSep Cape-Open
Property Packages are used: The SRK-UMR is used to describe the VLE in the high pressure reactor section and the gamma-phi method with NRTL parameters predicted from UNIFAC is used for
the vacuum distillation of the ethanolamines.
|
| [fsd] [png] |
 Ethyl-Benzene Styrene Monomer process (4/4/2021).
Combined Ethyl-Benzene (EB) Styrene Monomer (SM) process as shown in the 2005 AIChE Spring Meeting presentation "Design Guidelines for Distillation Columns in Ethyl-benzene and Styrene Monomer Service" by Peter Faessler et al. who discuss the Badger-SM process with details about revamping of the EB/SM splitter with high capacity structured packing for increasing performance specifciations. The flowsheet features the use of different ChemSep Cape-Open Property Packages (ChemSep/Copp) for the high and low pressure operations.
Ethyl-Benzene Styrene Monomer process (4/4/2021).
Combined Ethyl-Benzene (EB) Styrene Monomer (SM) process as shown in the 2005 AIChE Spring Meeting presentation "Design Guidelines for Distillation Columns in Ethyl-benzene and Styrene Monomer Service" by Peter Faessler et al. who discuss the Badger-SM process with details about revamping of the EB/SM splitter with high capacity structured packing for increasing performance specifciations. The flowsheet features the use of different ChemSep Cape-Open Property Packages (ChemSep/Copp) for the high and low pressure operations.
|
| [fsd] [png] |
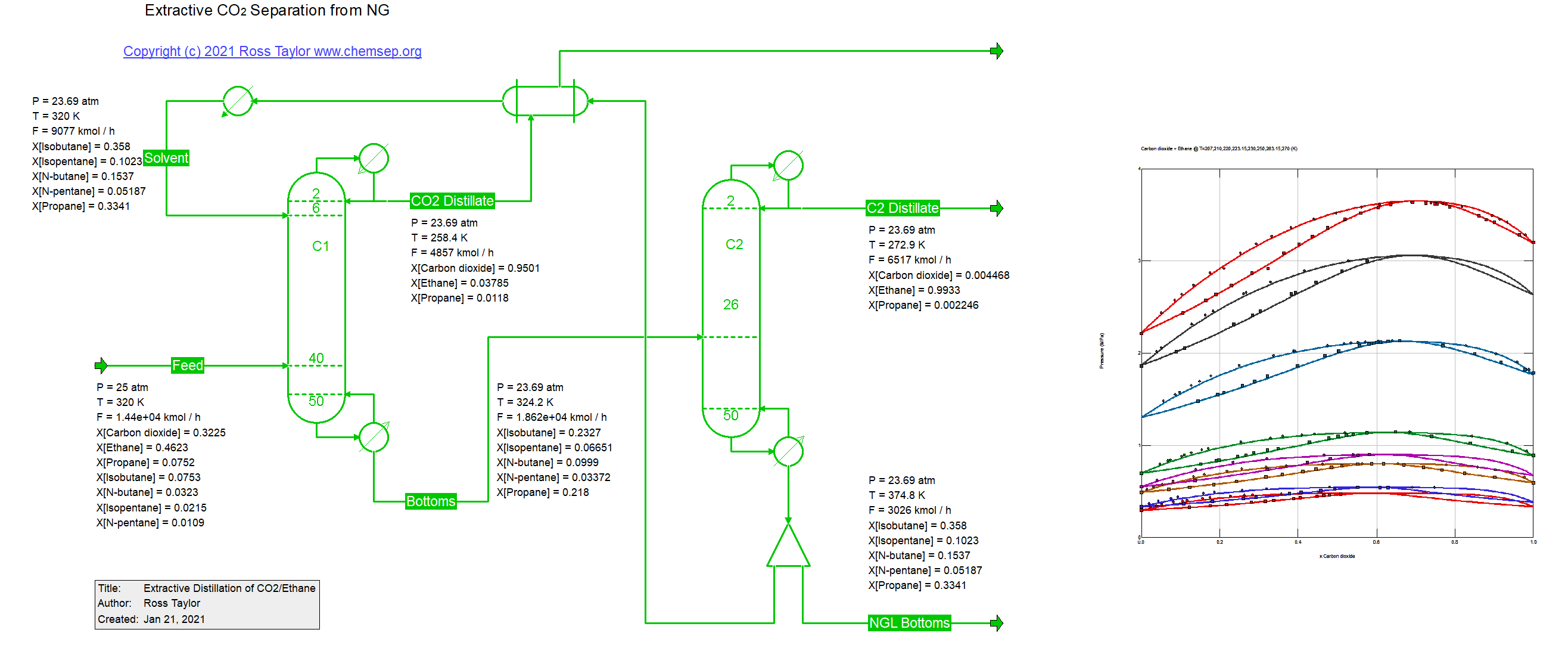 Extraction separation of CO2 from NG (24/1/2021).
For many years CO2 has been injected into oil and gas wells in an effort to extract more crude oil and natural gas. After methane has been
separated from the CO2-rich gas in a demethanizer the CO2 needs to be recovered so that it can be re-injected into the well.
The separation of CO2 from the ethane rich fluid is complicated because of an azeotrope that exists between CO2 and ethane. One approach to
breaking azeotropes is via extractive distillation. In the process in this document the solvent is a mixture of the higher molecular weight
hydrocarbons. This flowsheet was adapted from one described by W.L. Luyben. Ours uses the PPR78 EOS which employs a group contribution
method for the estimation of temperature dependent binary interaction parameters.
Luyben, W. L. Control of an Extractive Distillation System for the Separation of CO2 and Ethane in Enhanced Oil Recovery Processes
Ind.Eng.Chem.Res. (2013) 52 pp. 10780-10787.
Extraction separation of CO2 from NG (24/1/2021).
For many years CO2 has been injected into oil and gas wells in an effort to extract more crude oil and natural gas. After methane has been
separated from the CO2-rich gas in a demethanizer the CO2 needs to be recovered so that it can be re-injected into the well.
The separation of CO2 from the ethane rich fluid is complicated because of an azeotrope that exists between CO2 and ethane. One approach to
breaking azeotropes is via extractive distillation. In the process in this document the solvent is a mixture of the higher molecular weight
hydrocarbons. This flowsheet was adapted from one described by W.L. Luyben. Ours uses the PPR78 EOS which employs a group contribution
method for the estimation of temperature dependent binary interaction parameters.
Luyben, W. L. Control of an Extractive Distillation System for the Separation of CO2 and Ethane in Enhanced Oil Recovery Processes
Ind.Eng.Chem.Res. (2013) 52 pp. 10780-10787.
|
| [fsd] [png] |
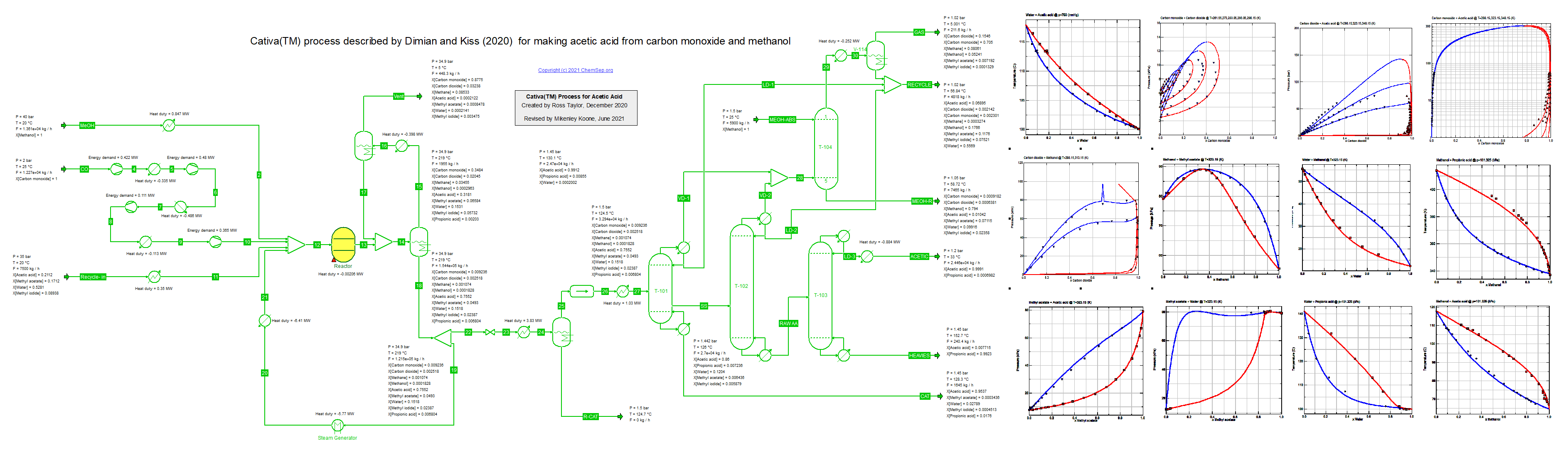 This flowheet was inspired by the article of Dimian and Kiss on the Acetic Acid Cativa(TM) process (21/2/2021) for making acetic acid from
carbon monoxide and methanol, see Chem.Eng.Res.Des. (2020) 159, pp. 1-12.
The recycle is estimated; neither Dimian and Kiss nor we included the necessary recovery units in the model.
This flowsheet examplifies the use of two different K-models: PSRK equation of state for the high pressure reactor section and
gamma-phi model for the separation section where the difficult separation between acetic acid and water is key.
The binary interaction parameters have been fit to data for most of the nonideal binary mixtures found in this process.
There is scope for further improvement in modeling the solubility of the light gases in the separaton section;
here we adopted the approach of Prausnitz et al. (1980).
This flowheet was inspired by the article of Dimian and Kiss on the Acetic Acid Cativa(TM) process (21/2/2021) for making acetic acid from
carbon monoxide and methanol, see Chem.Eng.Res.Des. (2020) 159, pp. 1-12.
The recycle is estimated; neither Dimian and Kiss nor we included the necessary recovery units in the model.
This flowsheet examplifies the use of two different K-models: PSRK equation of state for the high pressure reactor section and
gamma-phi model for the separation section where the difficult separation between acetic acid and water is key.
The binary interaction parameters have been fit to data for most of the nonideal binary mixtures found in this process.
There is scope for further improvement in modeling the solubility of the light gases in the separaton section;
here we adopted the approach of Prausnitz et al. (1980).
|
| [fsd] [png] |
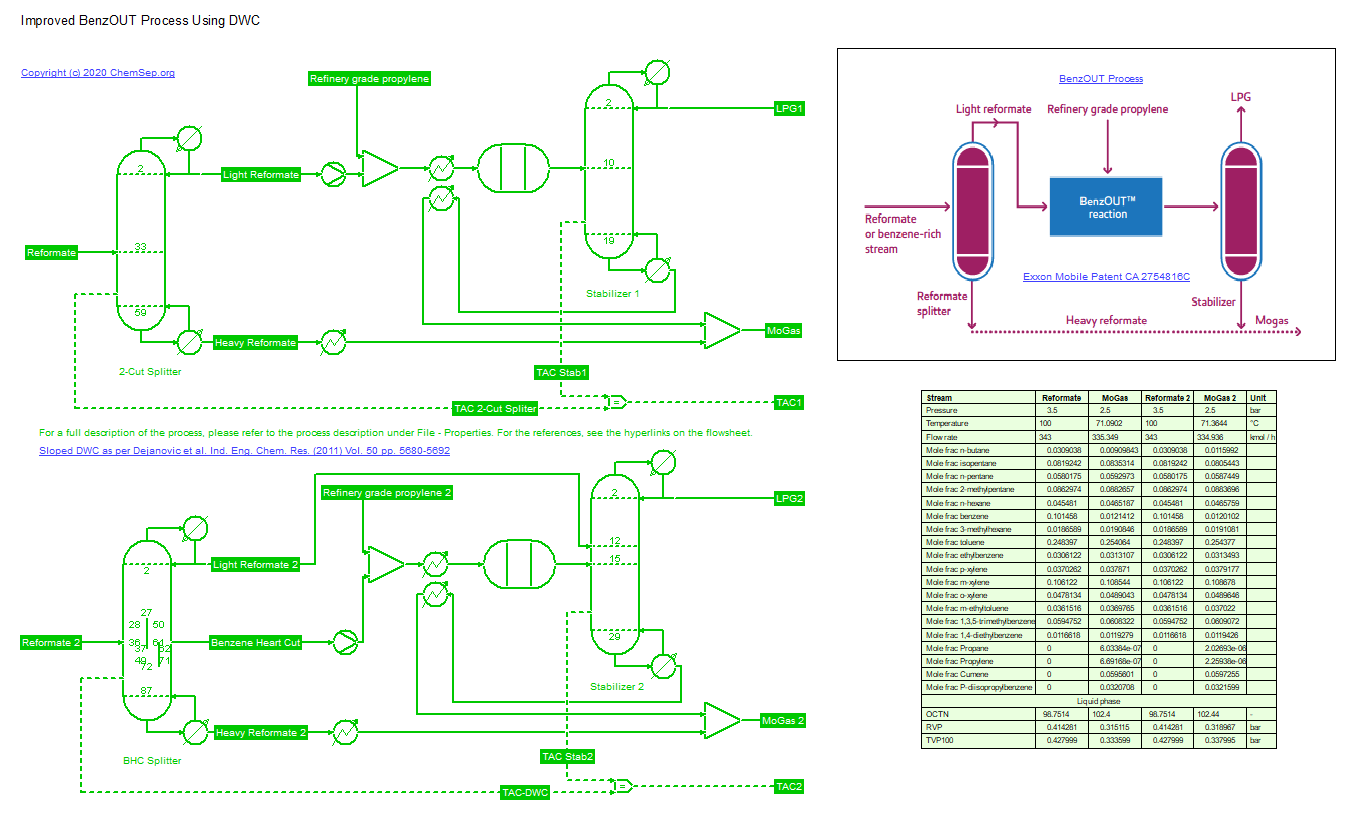 Improved BenzOut Process (29/12/2020) using a Dividing Wall Column (with sloped wall) with enhanced product recovery. This process was developed by ExxonMobile (CA2754816C) and features a zeolite fixed bed
liquid reactor operating at low temperatures where the Benzene in reformate from a CCR is converted for 95% to alkylbenzenes by reacting it with refinery grade (95%) Propylene from a FCC unit.
This generates a Mogas blending stream with a higher octane number (by 2-3 points on RON/MON) that is within Benzene specification. As a by-product Propane of HD5 quality is generated.
By combining this process with the Dividing Wall Column (DWC) as disclosed by Dejanovic et al. as described in Ind. Eng. Chem. Res. (2011) Vol. 50 pp. 5680-5692 (with sloped wall DWC)
an approximate 40% reduction in Total Annulized Cost and a 5% larger product flowrate can be obtained. The DWC produces a heart-cut Benzene rich stream that results in smaller reactors
that can be run with a 99% conversion. The resulting stream is stabilized and mixed with the heavy reformate stream.
Improved BenzOut Process (29/12/2020) using a Dividing Wall Column (with sloped wall) with enhanced product recovery. This process was developed by ExxonMobile (CA2754816C) and features a zeolite fixed bed
liquid reactor operating at low temperatures where the Benzene in reformate from a CCR is converted for 95% to alkylbenzenes by reacting it with refinery grade (95%) Propylene from a FCC unit.
This generates a Mogas blending stream with a higher octane number (by 2-3 points on RON/MON) that is within Benzene specification. As a by-product Propane of HD5 quality is generated.
By combining this process with the Dividing Wall Column (DWC) as disclosed by Dejanovic et al. as described in Ind. Eng. Chem. Res. (2011) Vol. 50 pp. 5680-5692 (with sloped wall DWC)
an approximate 40% reduction in Total Annulized Cost and a 5% larger product flowrate can be obtained. The DWC produces a heart-cut Benzene rich stream that results in smaller reactors
that can be run with a 99% conversion. The resulting stream is stabilized and mixed with the heavy reformate stream.
|
| [fsd] [png] |
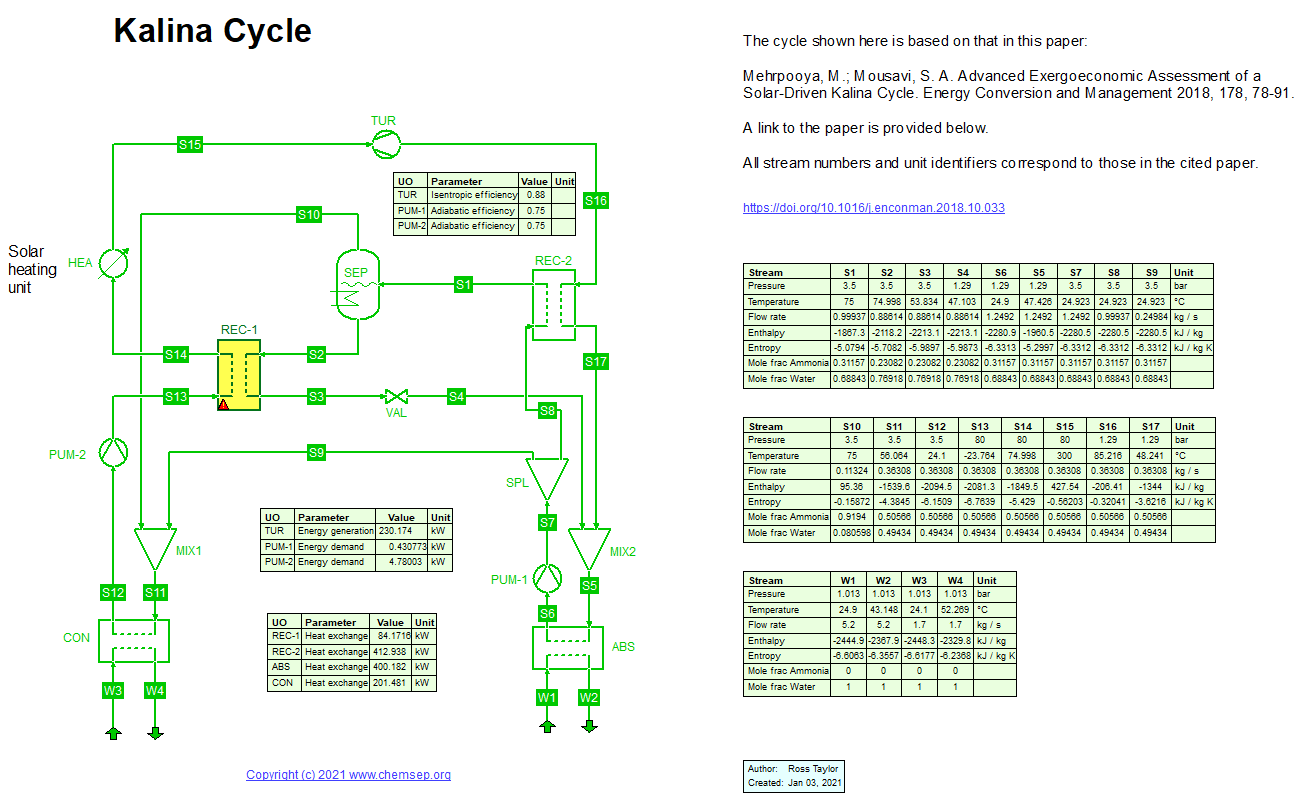 Exergy and economic assessment of a solar-driven Kalina cycle (4/1/2021) with Ammonia-Water as per Mehrpooya and Mousavi in
Energy Conversion and Management (2018) 178, pp. 78-91
Exergy and economic assessment of a solar-driven Kalina cycle (4/1/2021) with Ammonia-Water as per Mehrpooya and Mousavi in
Energy Conversion and Management (2018) 178, pp. 78-91
|
| [fsd] [fsd] [fsd] [fsd] [fsd] [fsd] [png] |
 Refrigeration cycles with Ammonia, Propylene, 2-step Propylene+Ethylene, 3-step Propylene+Ethylene+Methane, and
4-step Propylene+Ethylene+Methane+Nitrogen for cooling at -30, -50, -100, -150, and -190 C as described by Luyben (2019) in
Chem.Eng.Process.Intens. 138 (2019) pp. 97-110 and
Comp.Chem.Engng 126 (2019) pp. 241-248 (17/1/2021).
Refrigeration cycles with Ammonia, Propylene, 2-step Propylene+Ethylene, 3-step Propylene+Ethylene+Methane, and
4-step Propylene+Ethylene+Methane+Nitrogen for cooling at -30, -50, -100, -150, and -190 C as described by Luyben (2019) in
Chem.Eng.Process.Intens. 138 (2019) pp. 97-110 and
Comp.Chem.Engng 126 (2019) pp. 241-248 (17/1/2021).
|
| [fsd] [png] |
 Ethylene Oxide Process as per US 7,598,405 (18/1/2021) co-producing high purity Ethylene Oxide (EO) and Ethylene Glycol (EG). The reactor was setup around Start of Run (SOR)
data on inlet and outlet mass rates provided in the 2015 PhD thesis by Samir C. Nimkar on the Exergy Analysis of industrial chemical processes. At SOR the reactor outlet
temperature is 233 C. At 20.5 bar the high activity catalyst reaches an initial selectivity of 81% and 12.2% Ethylene conversion per pass. Conversion of undesired by-products
Acetaldehyde and Formaldehyde were set to 0.1% and 0.01%, respectively (as per 2012 PhD thesis of Madhav Ghanta). The simulation here is setup for an EO production of
just 1 t/h whereas real industrial reactors are sized to produce anywhere from 50 to 150 t/h.
Ethylene Oxide Process as per US 7,598,405 (18/1/2021) co-producing high purity Ethylene Oxide (EO) and Ethylene Glycol (EG). The reactor was setup around Start of Run (SOR)
data on inlet and outlet mass rates provided in the 2015 PhD thesis by Samir C. Nimkar on the Exergy Analysis of industrial chemical processes. At SOR the reactor outlet
temperature is 233 C. At 20.5 bar the high activity catalyst reaches an initial selectivity of 81% and 12.2% Ethylene conversion per pass. Conversion of undesired by-products
Acetaldehyde and Formaldehyde were set to 0.1% and 0.01%, respectively (as per 2012 PhD thesis of Madhav Ghanta). The simulation here is setup for an EO production of
just 1 t/h whereas real industrial reactors are sized to produce anywhere from 50 to 150 t/h.
|
| [fsd] [png] |
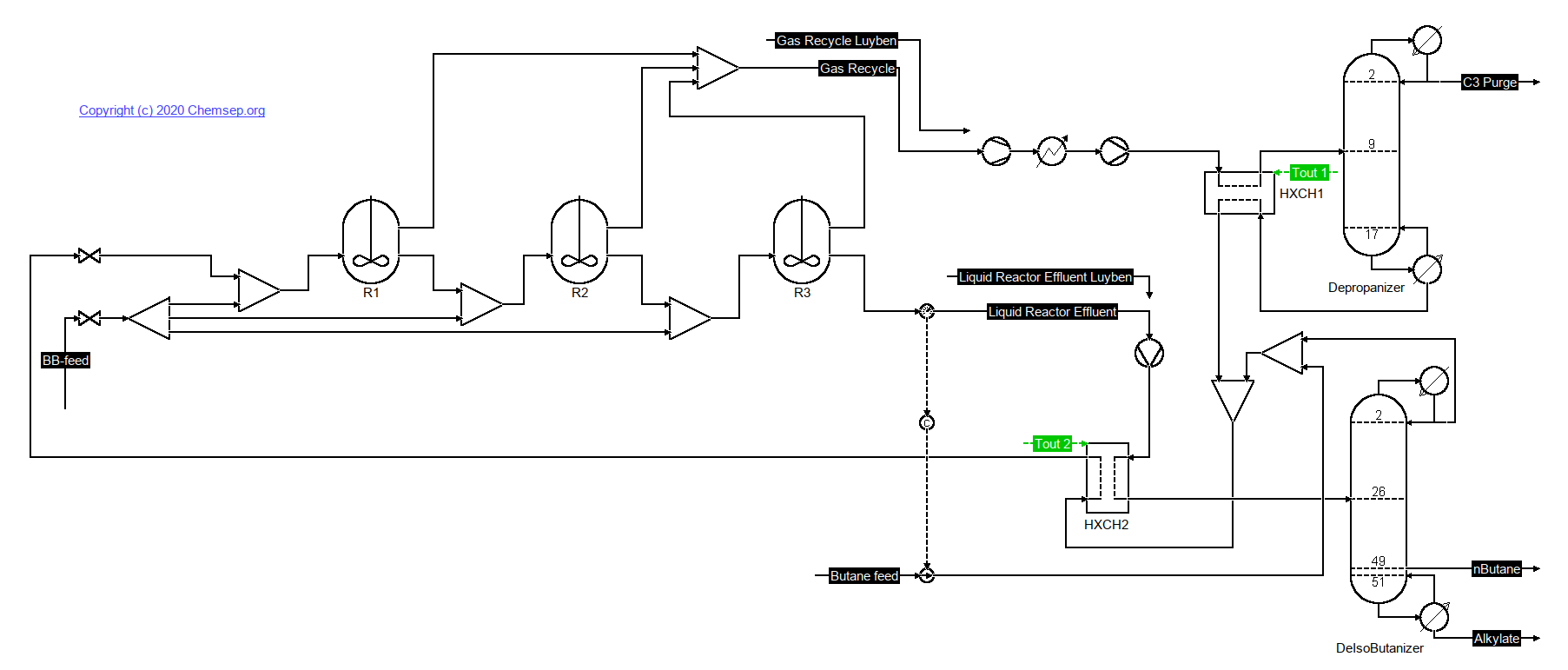 Alkylation of Butene and Isobutane (26/1/2021) as described by Luyben in Principles and Case Studies of Simultaneous Design (Wiley, 2011) and
Ind. Eng. Chem. Res. (2009) 48, p. 11081.
This flowsheet implements a simplification of the Kellogg Sulfuric Acid Butene-Butane Alkylation Process, as adapted from figure 7.2 in Chapter 7.
Reaction parameters from R. Mahajanam, R.V. Zheng, J.M. Douglas, "A shortcut method for control variable section and its application to the butane alkylation process",
Ind. Eng. Chem. Res. (2001) 40, p. 3208.
Note: To converge from a resetted flowsheet you need to hook-up both the gas and liquid recycle flows as given in Luyben.
After convergence is obtained you can then link-up the liquid recycle first, and solve again.
After converging the flowsheet again the gas recycle can also be linked-up.
Alkylation of Butene and Isobutane (26/1/2021) as described by Luyben in Principles and Case Studies of Simultaneous Design (Wiley, 2011) and
Ind. Eng. Chem. Res. (2009) 48, p. 11081.
This flowsheet implements a simplification of the Kellogg Sulfuric Acid Butene-Butane Alkylation Process, as adapted from figure 7.2 in Chapter 7.
Reaction parameters from R. Mahajanam, R.V. Zheng, J.M. Douglas, "A shortcut method for control variable section and its application to the butane alkylation process",
Ind. Eng. Chem. Res. (2001) 40, p. 3208.
Note: To converge from a resetted flowsheet you need to hook-up both the gas and liquid recycle flows as given in Luyben.
After convergence is obtained you can then link-up the liquid recycle first, and solve again.
After converging the flowsheet again the gas recycle can also be linked-up.
|
| [fsd] [png] |
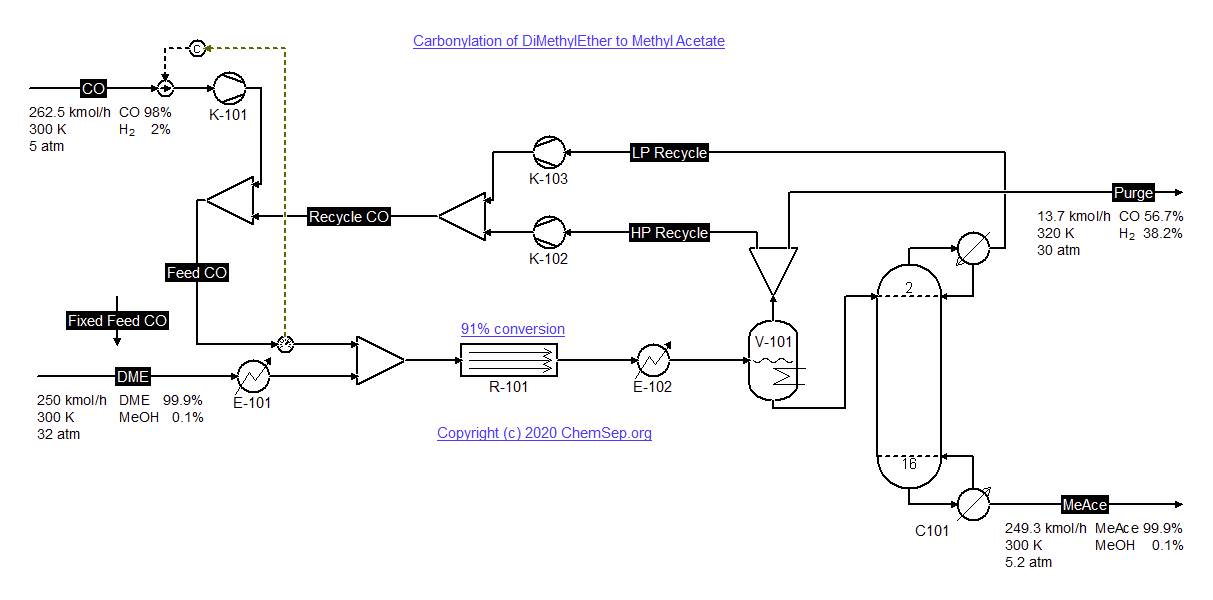 Carbonylation of Di-Methyl Ether with CO to Methyl Acetate (19/8/2020) by Diemer and Luyben
in Ind.Chem.Eng.Res.Des. Vol. 49 page 12224-12241.
Dimethyl ether (DME) is produced by dehydration of Methanol. In a second step DME is carbonylized with CO over zeolites to
produce Methyl Acetate (for kinetics see
Cheung et al. Angew.Chem.Int.Ed. (2006) 45, pp. 1617-1620
The process illustrates a number of important design trade-offs among the many design optimization variables: reactor temperatures,
reactor pressures, distillation column pressures, reactor sizes and purge composition.
Carbonylation of Di-Methyl Ether with CO to Methyl Acetate (19/8/2020) by Diemer and Luyben
in Ind.Chem.Eng.Res.Des. Vol. 49 page 12224-12241.
Dimethyl ether (DME) is produced by dehydration of Methanol. In a second step DME is carbonylized with CO over zeolites to
produce Methyl Acetate (for kinetics see
Cheung et al. Angew.Chem.Int.Ed. (2006) 45, pp. 1617-1620
The process illustrates a number of important design trade-offs among the many design optimization variables: reactor temperatures,
reactor pressures, distillation column pressures, reactor sizes and purge composition.
|
| [fsd] [png] |
 Extractive Distillation of Di-Methyl Carbonate (DMC) and Methanol (6/4/2020) using four different solvents as described in
Ind.Chem.Eng.Res.Des. Vol. 127 page 189.
DMC forms an azeotrope with Methanol. The paper discusses a holistic, three-tiered approach to find alternative extractive distillation entrainers via
a preliminary entrainer screening, measurement of limiting activity coefficients via headspace gas chromatography, and full binary Vapor-Liquid Equilibria measurements
using a dynamic recirculation cell. The effectiveness of the entrainers were then quantitatively assessed by simulation of the heat-integrated line-ups and
compared with that of the industrially used entrainer, Phenol. The simulation results reveal Methyl Salicylate as an MSA outperforms all other entrainers.
Extractive Distillation of Di-Methyl Carbonate (DMC) and Methanol (6/4/2020) using four different solvents as described in
Ind.Chem.Eng.Res.Des. Vol. 127 page 189.
DMC forms an azeotrope with Methanol. The paper discusses a holistic, three-tiered approach to find alternative extractive distillation entrainers via
a preliminary entrainer screening, measurement of limiting activity coefficients via headspace gas chromatography, and full binary Vapor-Liquid Equilibria measurements
using a dynamic recirculation cell. The effectiveness of the entrainers were then quantitatively assessed by simulation of the heat-integrated line-ups and
compared with that of the industrially used entrainer, Phenol. The simulation results reveal Methyl Salicylate as an MSA outperforms all other entrainers. This flowsheet illustrate how ChemSep can perform the fitting of the four new binary VLE data-sets to obtain UNIQUAC binary interaction parameters which are used in a ChemSep Cape-Open Property Package (CS/COPP) and how the sizing of the columns on the rating panel can directly provide the Total Annualized Cost (TAC) for each entrainer at the flowsheet level to optimize the process. |
| [fsd] [png] [MSH fsd] [MSH png] |
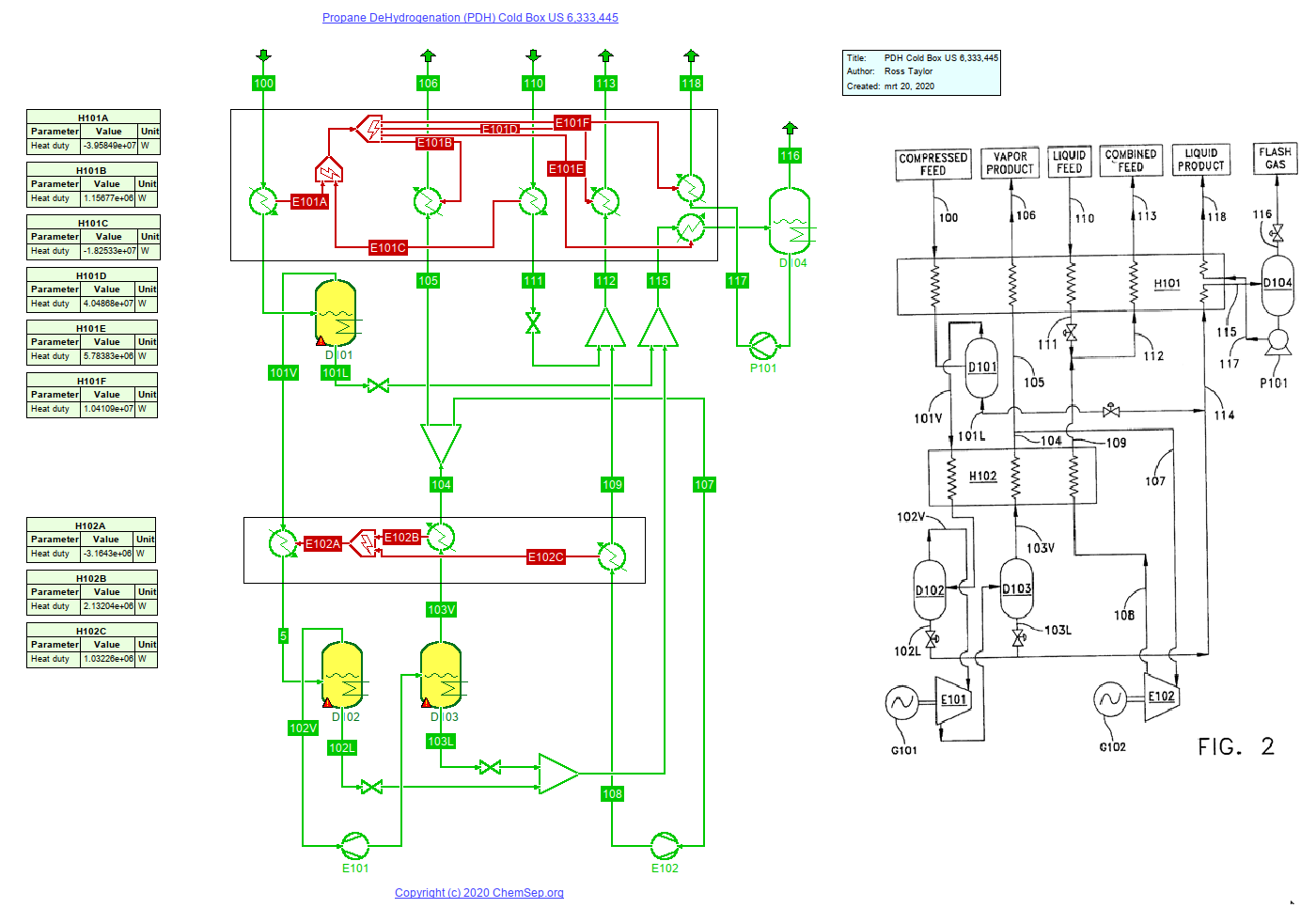
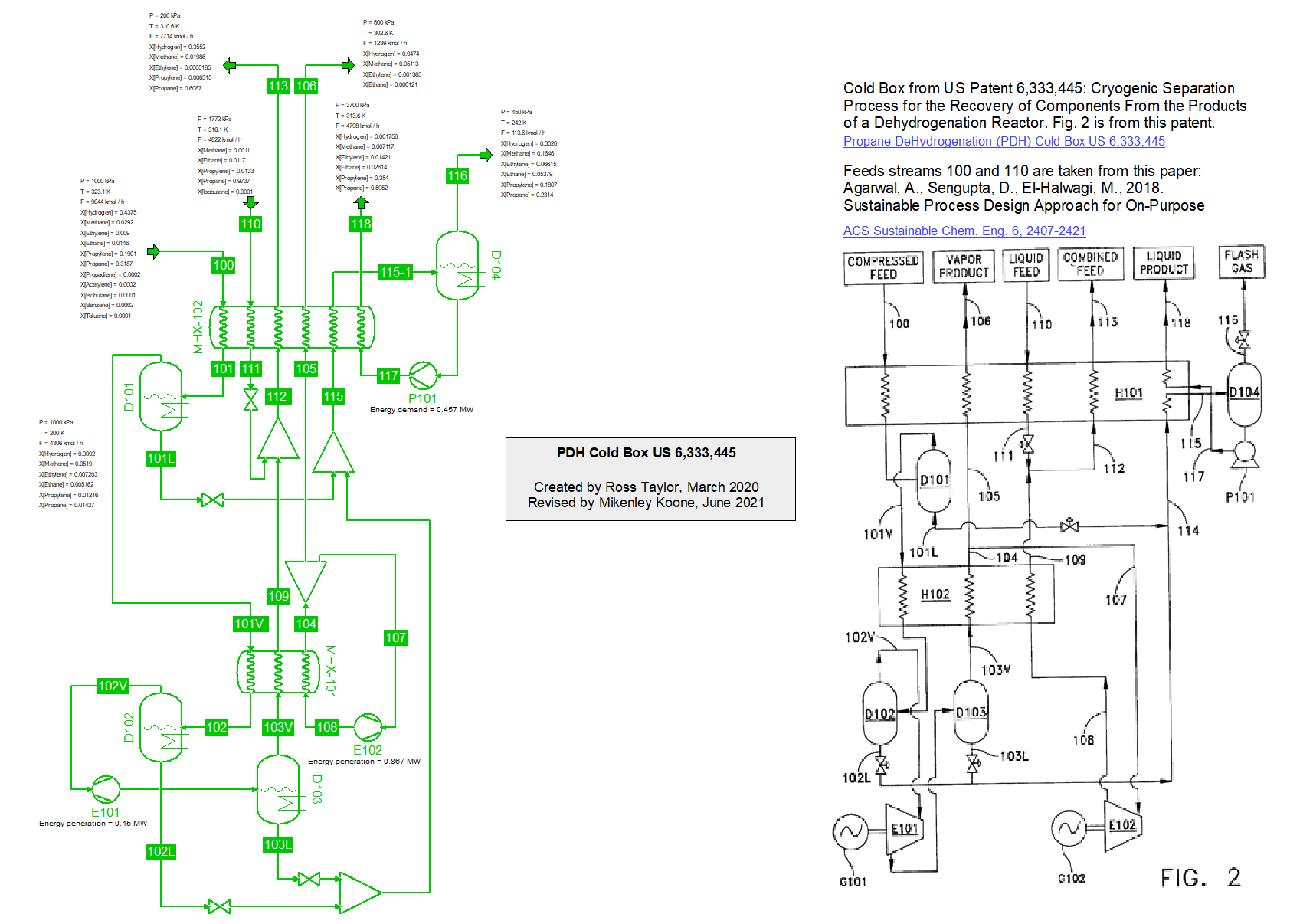 Cold box for the production of Propylene by Propane DeHydrogenation (PDH) from US 6,333,445 from Chart, Inc (21/2/2020).
Cold box for the production of Propylene by Propane DeHydrogenation (PDH) from US 6,333,445 from Chart, Inc (21/2/2020).
|
| [fsd] [png] |
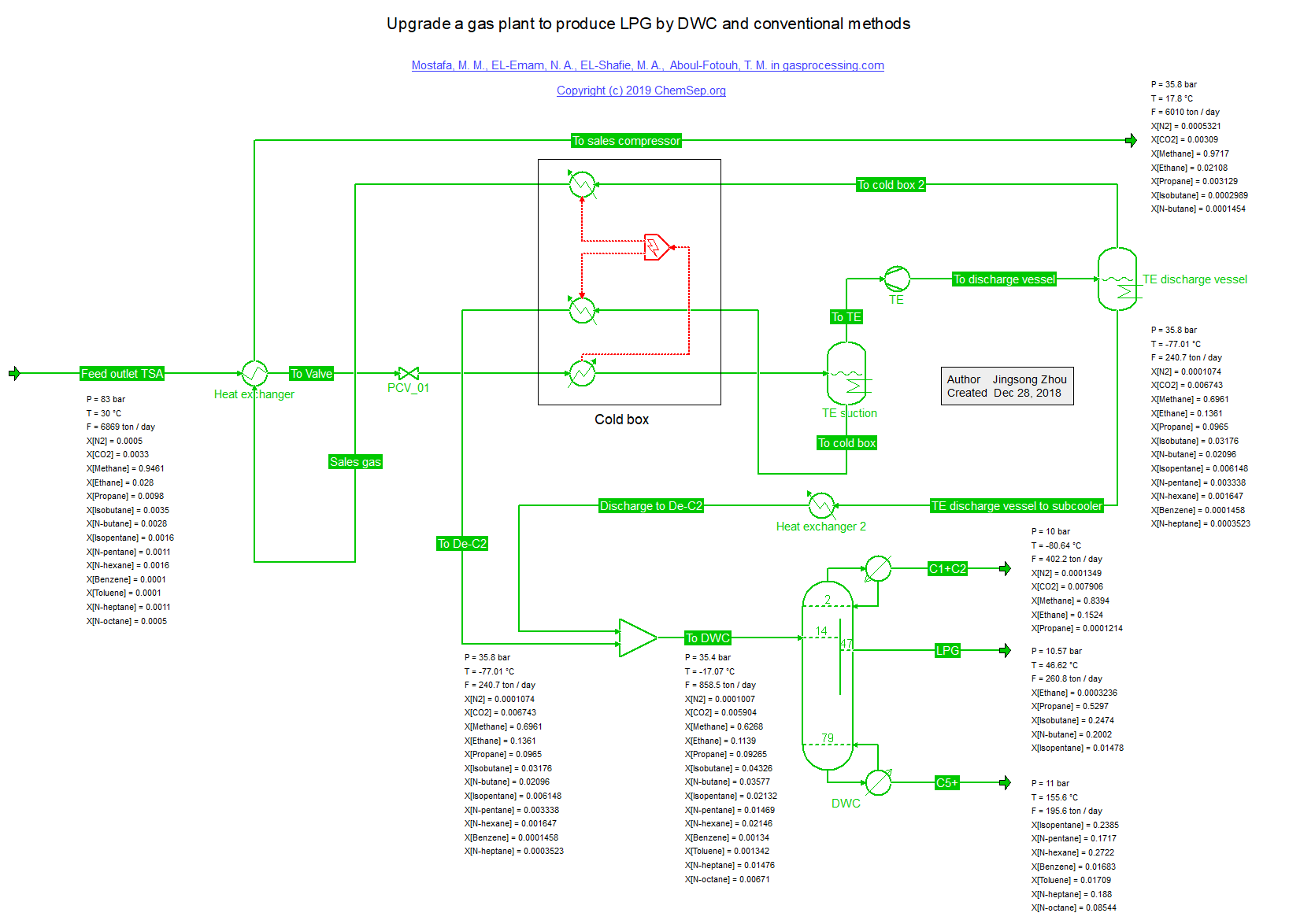 LPG Divided Wall Column (19/5/2019) for a lower cost separation of LPG from Natural Gas as described in
December 2018 Gas Processing issue.
LPG Divided Wall Column (19/5/2019) for a lower cost separation of LPG from Natural Gas as described in
December 2018 Gas Processing issue.
|
| [fsd] [fsd MSHX] [pdf] [png] |
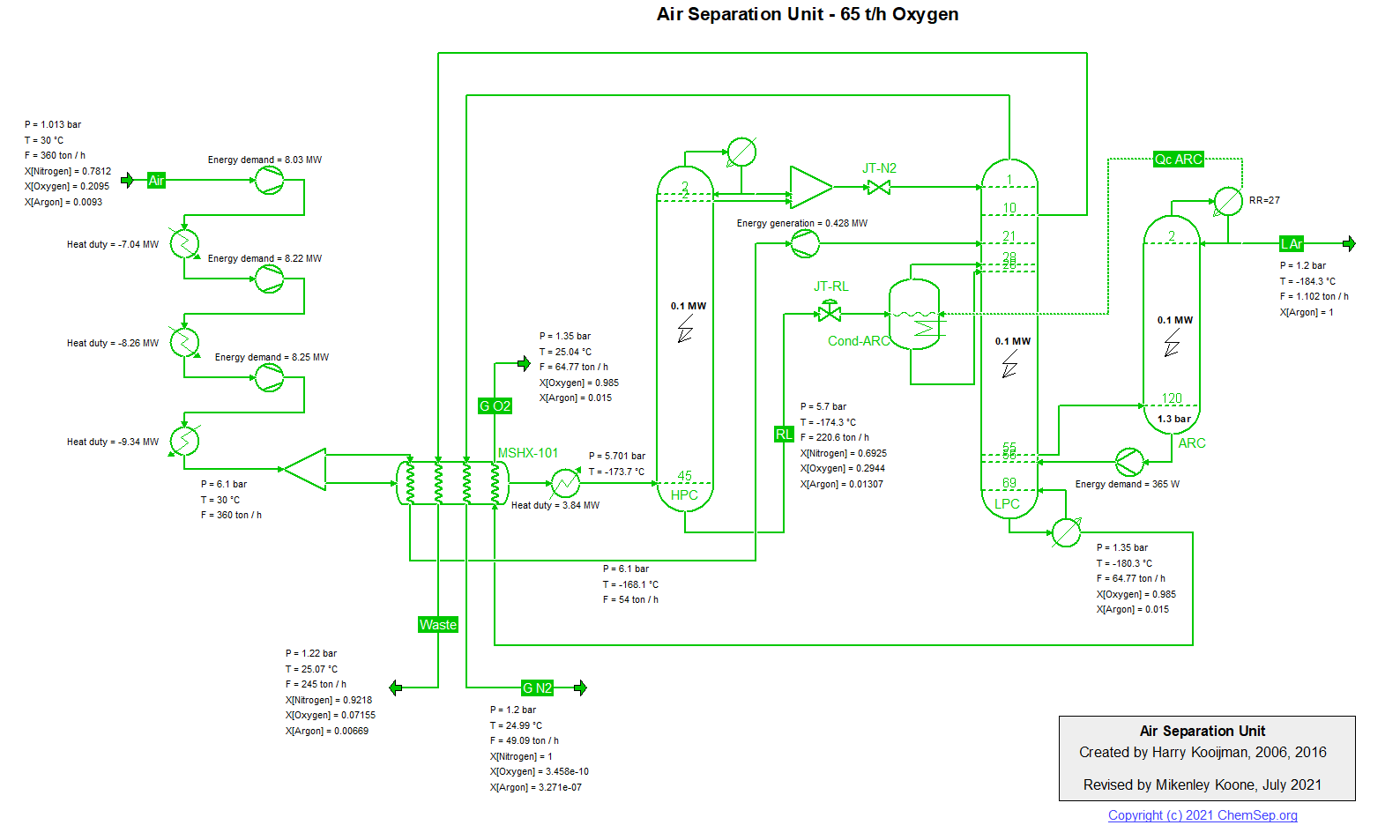
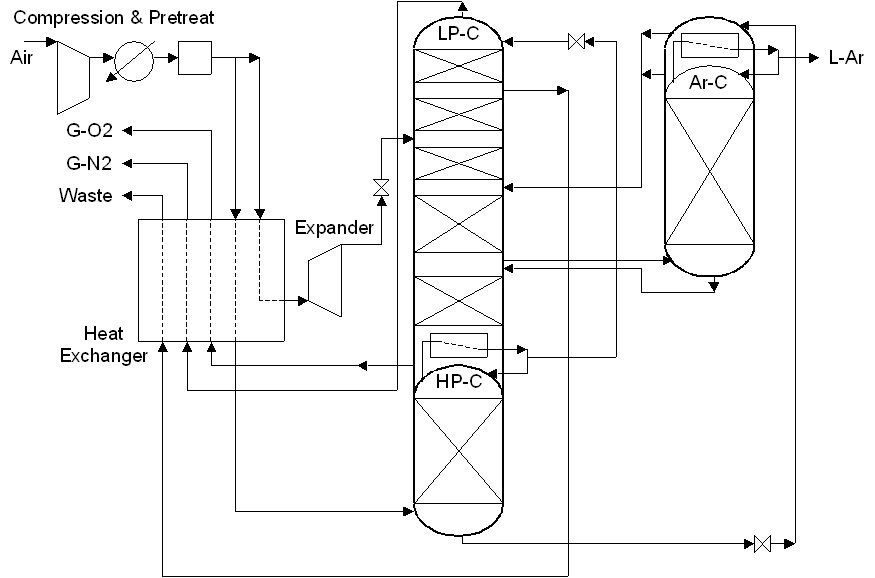 ASU (27/5/2018) flowsheet by H.Kooijman (original 2006).
Air Separation unit producing 60 t/h oxygen (recovery 75%) and liquid argon
(recovery 85%) using a simplified flowsheet (i.e. refrigeration air is not compressed to a higher pressure, resulting in a
lower efficiency, and the simple Peng-Robinson equation of state is used for simulation, leading to inaccuracies in the
predicted refrigeration due to the JT effect).
ASU (27/5/2018) flowsheet by H.Kooijman (original 2006).
Air Separation unit producing 60 t/h oxygen (recovery 75%) and liquid argon
(recovery 85%) using a simplified flowsheet (i.e. refrigeration air is not compressed to a higher pressure, resulting in a
lower efficiency, and the simple Peng-Robinson equation of state is used for simulation, leading to inaccuracies in the
predicted refrigeration due to the JT effect).
|
| [fsd] [png] |
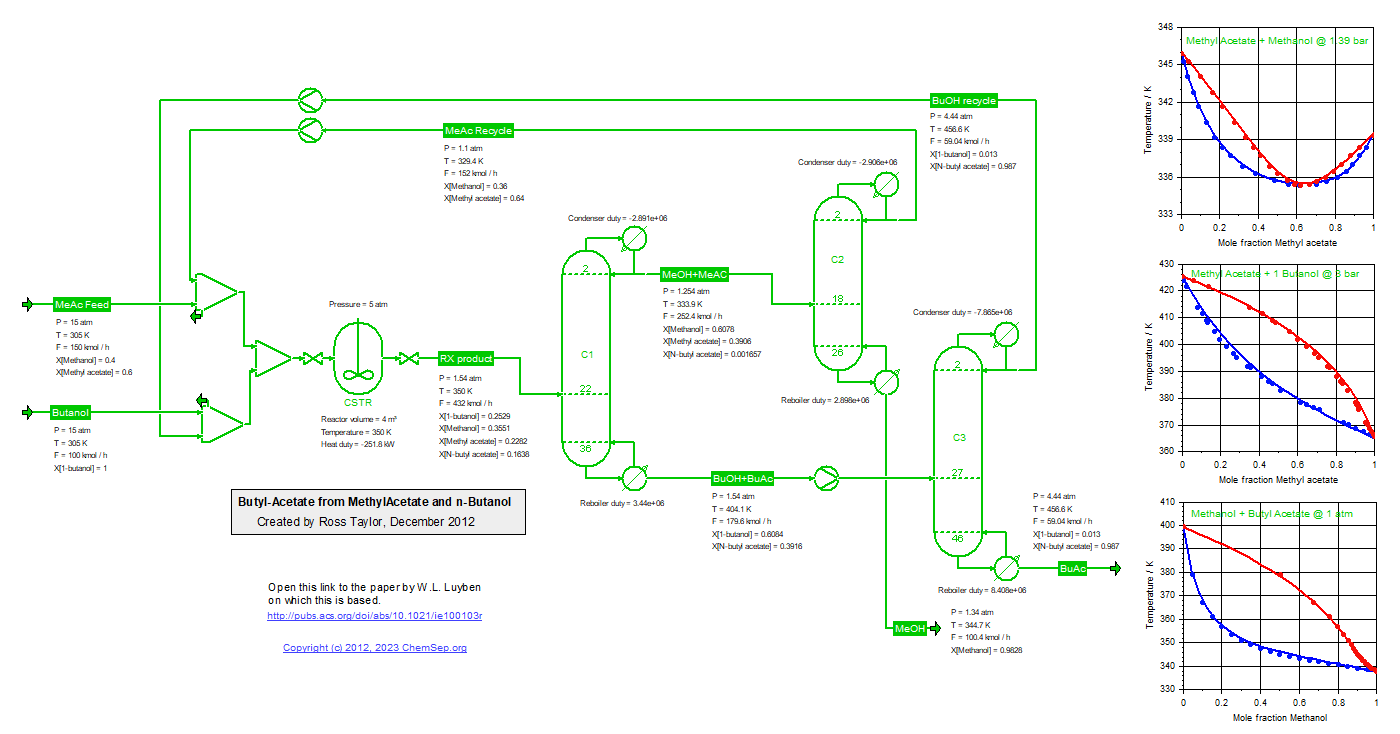 Process to synthesize Butyl Acetate from Methyl Acetate and Butanol (18/12/2011), adapted from Luyben et al.,
Ind.Eng.Chem.Res. (2011) 50 pp. 1247-1263.
Note that the temperature of the last column has been increased to 4.4 atm to match the bottom temperature
of the Butyl Acetate column. Also realize that the Methyl Acetate recycle rate is a strong function of the
chosen thermodynamic models and their interaction parameters.
Process to synthesize Butyl Acetate from Methyl Acetate and Butanol (18/12/2011), adapted from Luyben et al.,
Ind.Eng.Chem.Res. (2011) 50 pp. 1247-1263.
Note that the temperature of the last column has been increased to 4.4 atm to match the bottom temperature
of the Butyl Acetate column. Also realize that the Methyl Acetate recycle rate is a strong function of the
chosen thermodynamic models and their interaction parameters.
|
| [fsd] [png] |
 Process for producing Cumene from Benzene and Propylene (18/12/2011) as adapted by Luyben in
Ind.Eng.Chem.Res. (2010) Vol. 49 pp. 719-734.
Note that this flowsheet uses fixed conversion rates in the reactor whereas the original publication uses rate equations.
Process for producing Cumene from Benzene and Propylene (18/12/2011) as adapted by Luyben in
Ind.Eng.Chem.Res. (2010) Vol. 49 pp. 719-734.
Note that this flowsheet uses fixed conversion rates in the reactor whereas the original publication uses rate equations.
|
| [fsd] [png] |
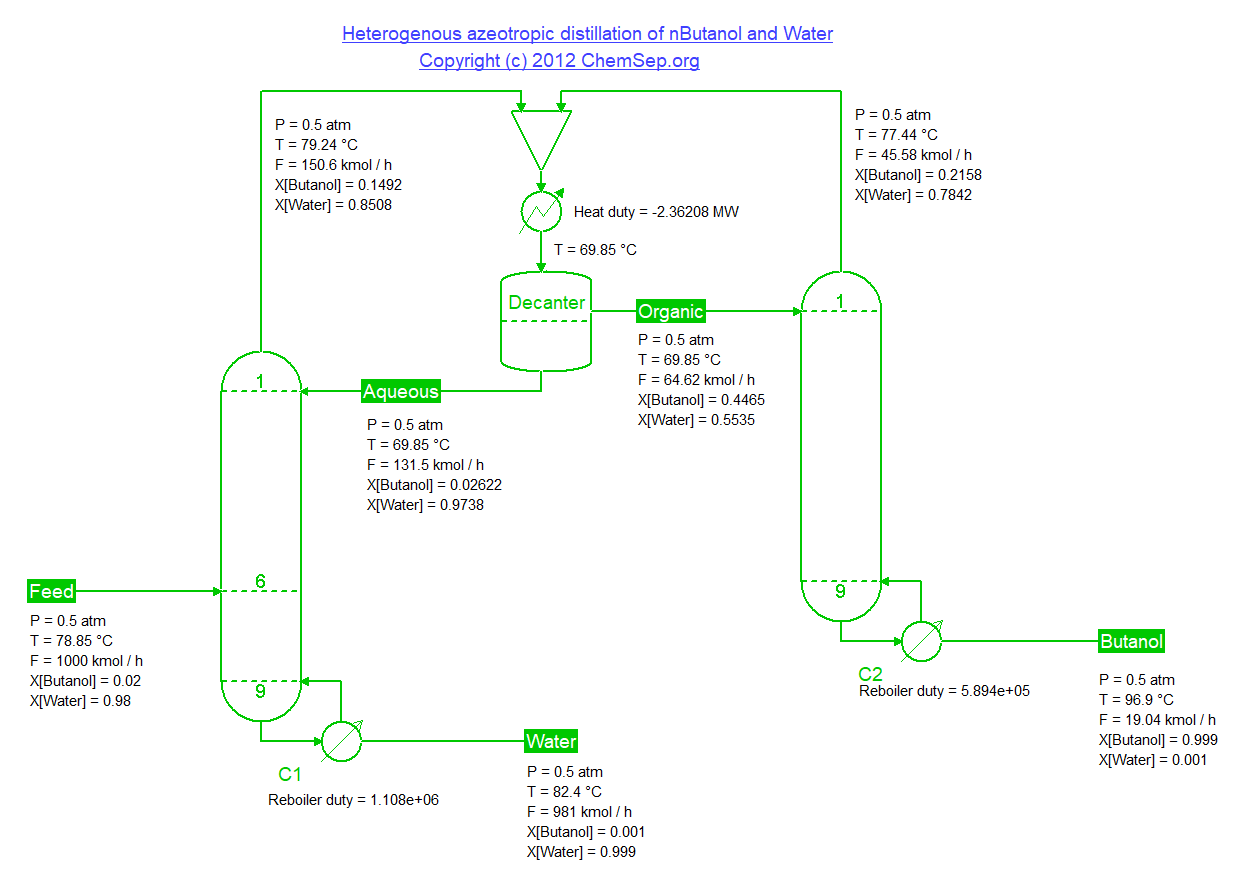 Separation of Butanol and Water by making use of the liquid-liquid-equilibria providing a means to break the vapor-liquid azeotrope,
adapted from Luyben et al. Energy Fuels (2008) 22 pp. 4249-4258.
Separation of Butanol and Water by making use of the liquid-liquid-equilibria providing a means to break the vapor-liquid azeotrope,
adapted from Luyben et al. Energy Fuels (2008) 22 pp. 4249-4258.
|
| [fsd] [png] |
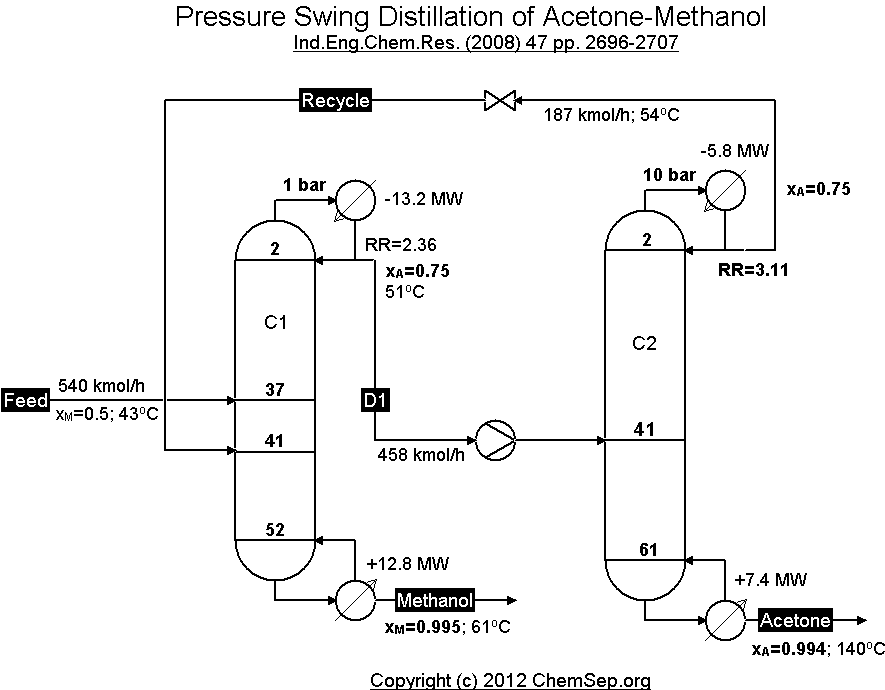 Separation of the Methanol and Acetone minimum temperature azeotrope by using the pressure sensitivity
of the azeotropic composition of this mixture by operating two columns at different pressures,
adapted from Luyben et al. Ind.Eng.Chem.Res. (2008) 47 pp. 2696-2707.
Separation of the Methanol and Acetone minimum temperature azeotrope by using the pressure sensitivity
of the azeotropic composition of this mixture by operating two columns at different pressures,
adapted from Luyben et al. Ind.Eng.Chem.Res. (2008) 47 pp. 2696-2707.
|
| [fsd] [png] |
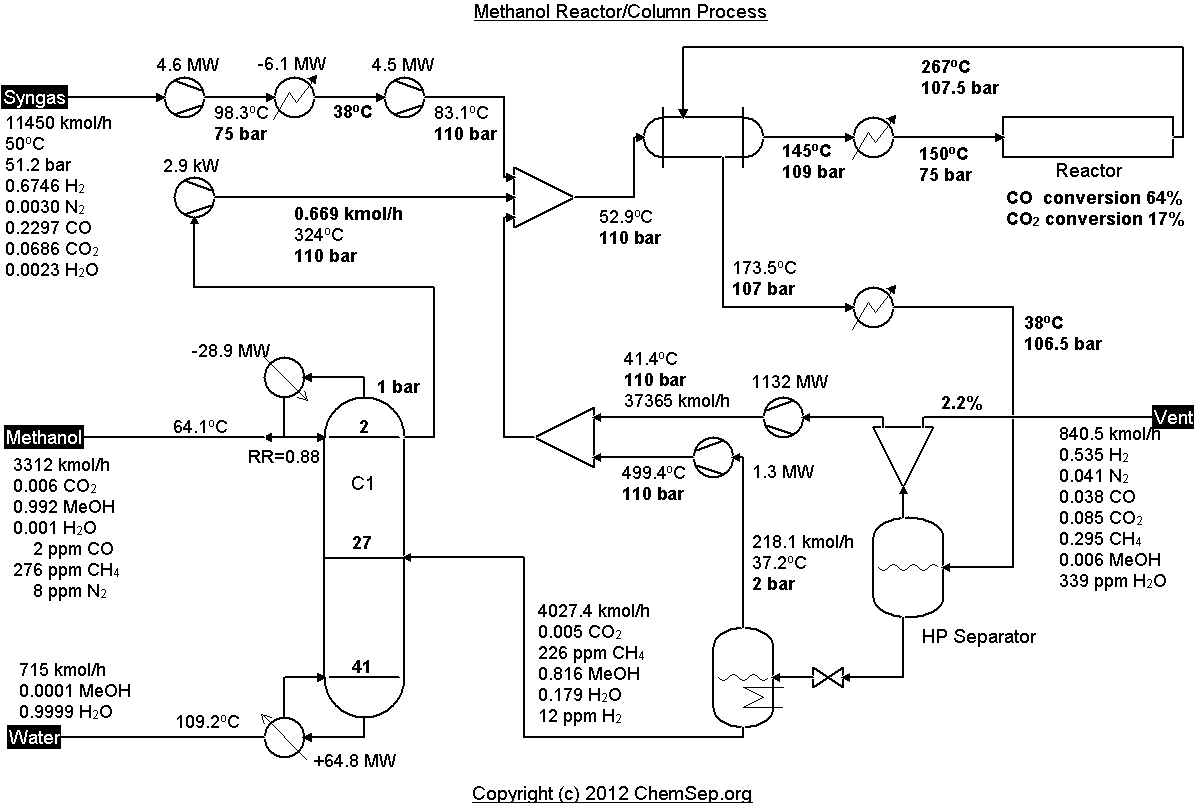 Methanol synthesis from syngas as described by Luyben et al.
Ind.Eng.Chem.Res. (2010) 49 pp. 6150-6163.
Note that this flowsheet uses fixed conversion rates in the reactor whereas the original publication uses rate equations.
Furthermore, the temperature of the vapor overhead recycle of the methanol column is highly dependent on the flowrate and
thermodynamic model selection. Adapted by Anders Andreaden with a plug-flow reactor using kinetics from Van-Dal and Bouallou.
For the distillation column the NRTL activity coefficient model is applied, whereas for the rest of the flowsheet the Peng-Robinson EOS is applied.
Methanol synthesis from syngas as described by Luyben et al.
Ind.Eng.Chem.Res. (2010) 49 pp. 6150-6163.
Note that this flowsheet uses fixed conversion rates in the reactor whereas the original publication uses rate equations.
Furthermore, the temperature of the vapor overhead recycle of the methanol column is highly dependent on the flowrate and
thermodynamic model selection. Adapted by Anders Andreaden with a plug-flow reactor using kinetics from Van-Dal and Bouallou.
For the distillation column the NRTL activity coefficient model is applied, whereas for the rest of the flowsheet the Peng-Robinson EOS is applied.
|
| [fsd] [png] [fsd] [png] |

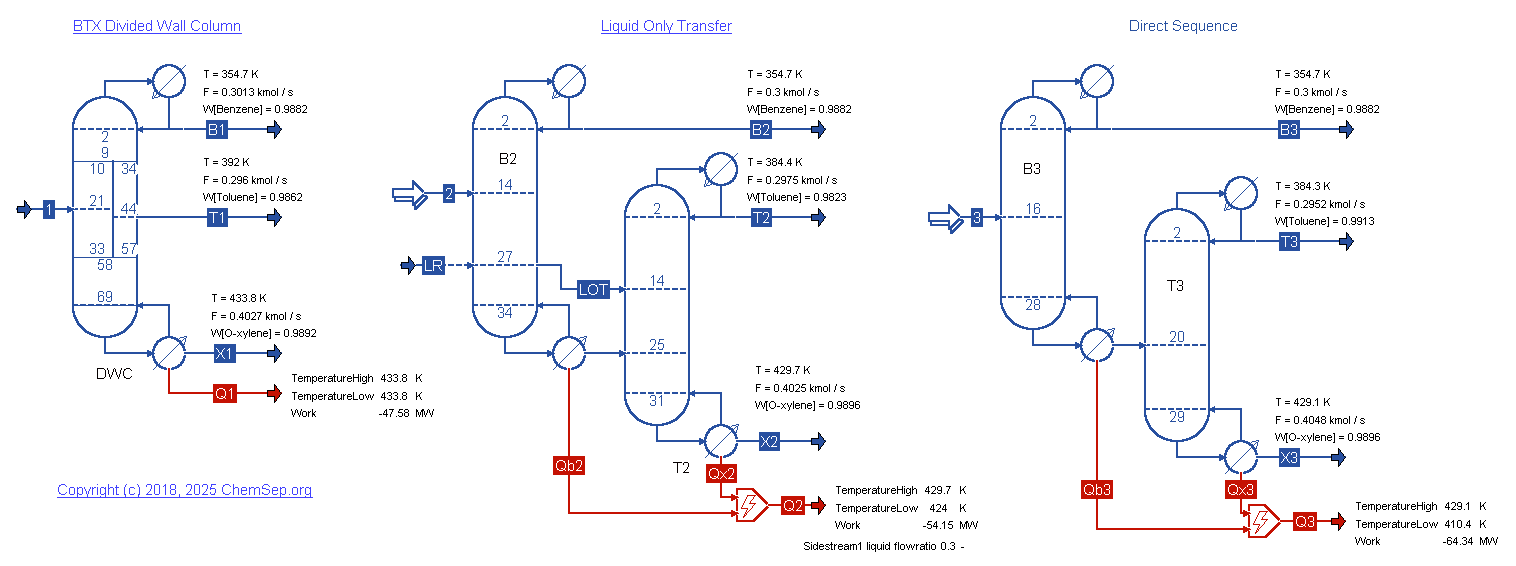 BTX Divided Wall Column as described by Luyben in Ind. Eng. Chem. Res. (2009) Vol. 48 pp. 6034-6049
simulated as one column.
A comparison between the DWC, Liquid Only Transfer (LOT), and the direct sequence shows that the DWC achieves a 30 percent savings in reboiler duty over a direct sequence.
Since the DWC only has a singlle condenser and reboiler, it has a similar 30 percent savings in CAPEX.
Liquid Only Transfers are described by Agrawal in AIChE J. (2000) Vol. 46 pp. 2198-2210).
For this BTX separation the LOT already accomplishes a savings in reboiler duty of 14 percent.
Since LOTs are very easy to implement in existing columns sequences they provide the quickest route to CO2 savings.
BTX Divided Wall Column as described by Luyben in Ind. Eng. Chem. Res. (2009) Vol. 48 pp. 6034-6049
simulated as one column.
A comparison between the DWC, Liquid Only Transfer (LOT), and the direct sequence shows that the DWC achieves a 30 percent savings in reboiler duty over a direct sequence.
Since the DWC only has a singlle condenser and reboiler, it has a similar 30 percent savings in CAPEX.
Liquid Only Transfers are described by Agrawal in AIChE J. (2000) Vol. 46 pp. 2198-2210).
For this BTX separation the LOT already accomplishes a savings in reboiler duty of 14 percent.
Since LOTs are very easy to implement in existing columns sequences they provide the quickest route to CO2 savings.
|
| [fsd] [png] |
 Separation of Ethanol and Water using pervaporization to break the azeotrope. Note that the reflux ratio is
set instead of the overhead composition because the sensitivity to the binary interaction parameters of the UNIQUAC
model and the vapor pressure models. Specification of an 85% overhead would lower the reflux ratio to 2.5, lowering
the condenser duty requirement.
Adapted from Luyben, Ind.Eng.Chem.Res. (2009) 48 pp. 3484-3495.
Separation of Ethanol and Water using pervaporization to break the azeotrope. Note that the reflux ratio is
set instead of the overhead composition because the sensitivity to the binary interaction parameters of the UNIQUAC
model and the vapor pressure models. Specification of an 85% overhead would lower the reflux ratio to 2.5, lowering
the condenser duty requirement.
Adapted from Luyben, Ind.Eng.Chem.Res. (2009) 48 pp. 3484-3495.
|
| [fsd] [png] |
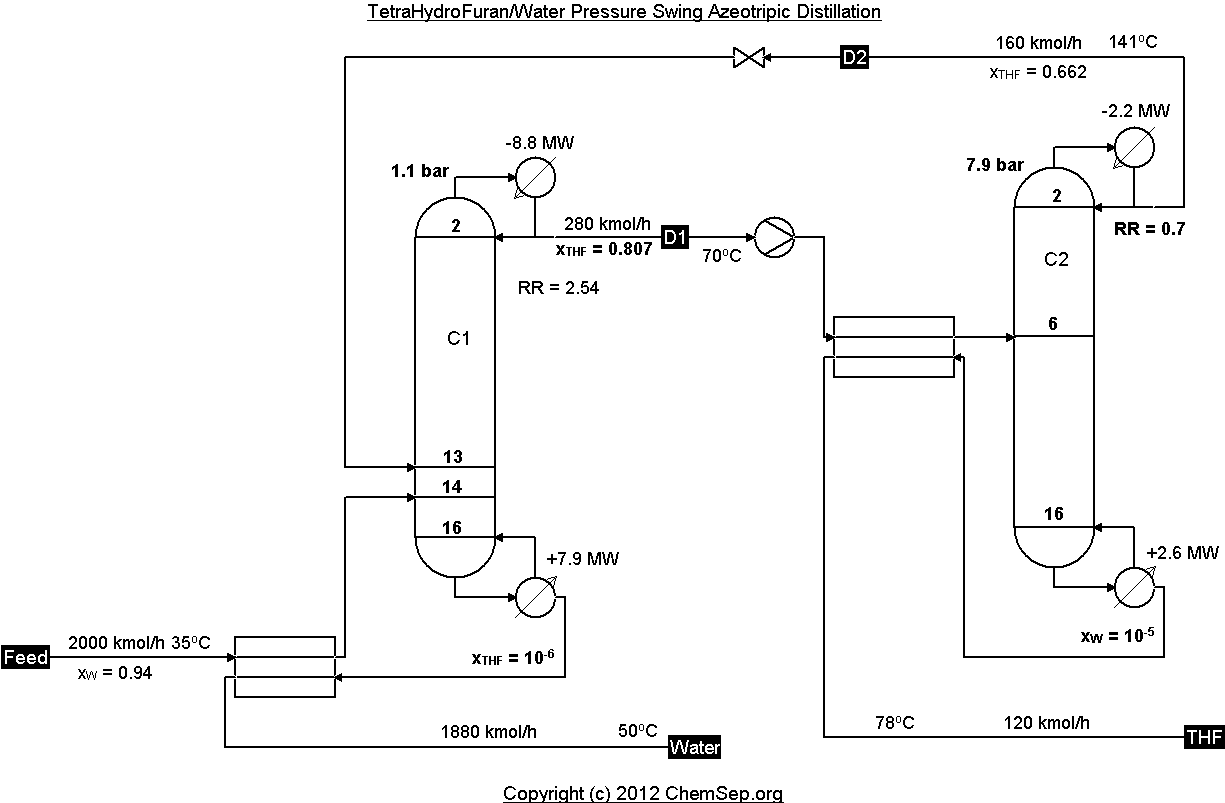 Pressure swing THF / Water azeotropic distillation with two columns operating at different pressures using
heat integration, as described by Luyben in Ind.Eng.Chem.Res. (2008) Vol. 47 pp. 2681-2695.
Pressure swing THF / Water azeotropic distillation with two columns operating at different pressures using
heat integration, as described by Luyben in Ind.Eng.Chem.Res. (2008) Vol. 47 pp. 2681-2695.
|
| [pdf] [fsd] [png] |
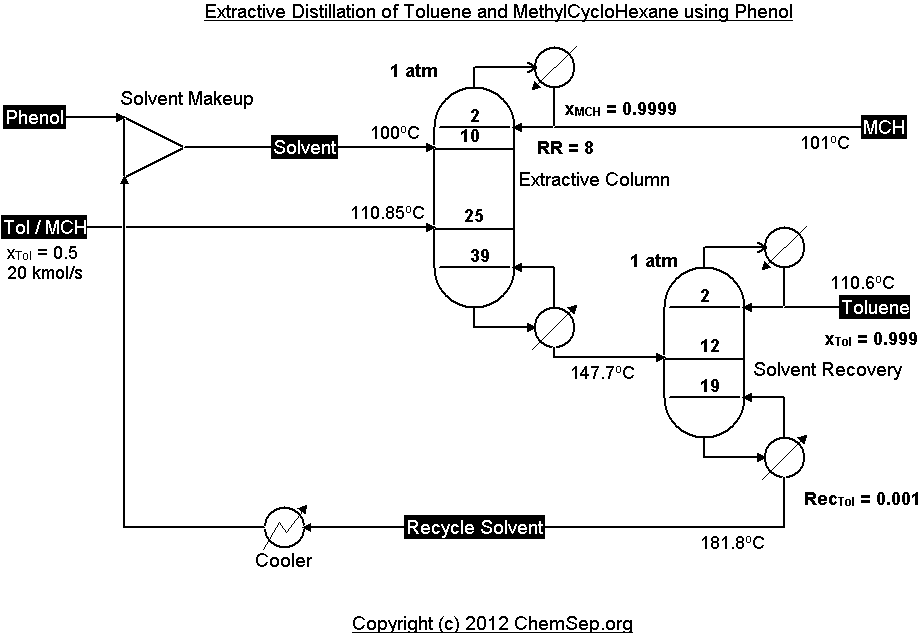 Extractive distillation of MethylCyloHexane/Toluene using Phenol adapted from Tiverios and Van Brunt in
Ind.Eng.Chem.Res. (2000) 39, pp. 1614-1623
Extractive distillation of MethylCyloHexane/Toluene using Phenol adapted from Tiverios and Van Brunt in
Ind.Eng.Chem.Res. (2000) 39, pp. 1614-1623
|
| [fsd] [png] |
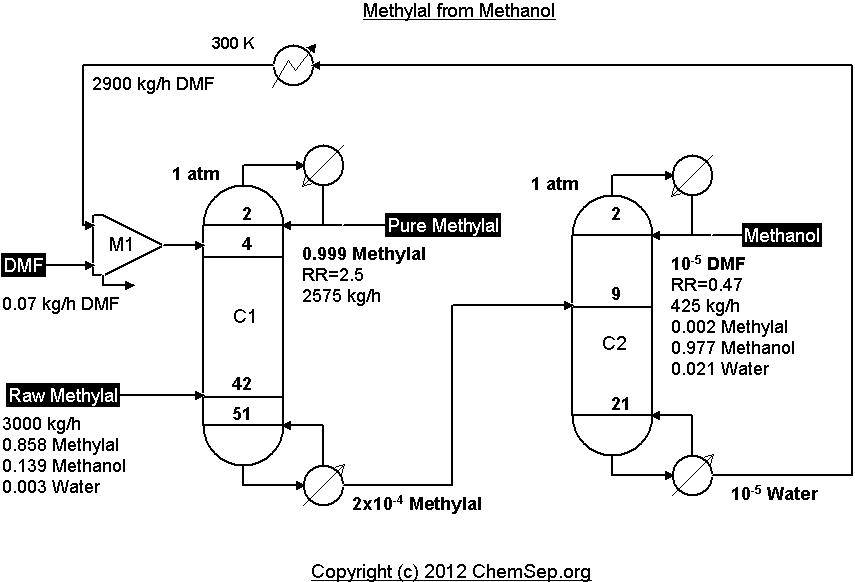 Extractive distillation of Methylal from Methanol using DMF as described by Wang et al. in
Ind.Eng.Chem.Res. (2012) Vol. 51 pp. 1281-1292.
Extractive distillation of Methylal from Methanol using DMF as described by Wang et al. in
Ind.Eng.Chem.Res. (2012) Vol. 51 pp. 1281-1292.
|
| [pdf] [sep] | Aromatics column described by R. Strigle (Gulf., 1987). |
| [pdf] [sep] | Depropanizer described by R. Strigle (Gulf., 1987) to recover propylene and propane from C4 and heavier hydrocarbons. |
| [pdf] [sep] | Azeotropic distillation column of Methanol / Isopropanol with Water by DeRosier. |
| [pdf] [sep] | industrial i-butane/n-butane splitter as reported by Klemola and Ilme . |
| [pdf] [sep] | How to model columns involving components that are not included in the databank. |
| [pdf] | How to add compounds to the ChemSep databanks. |
| [fsd] | Benzene/Toluene/p-Xylene separation train from ChemSep book. |
| [fsd] [png] |
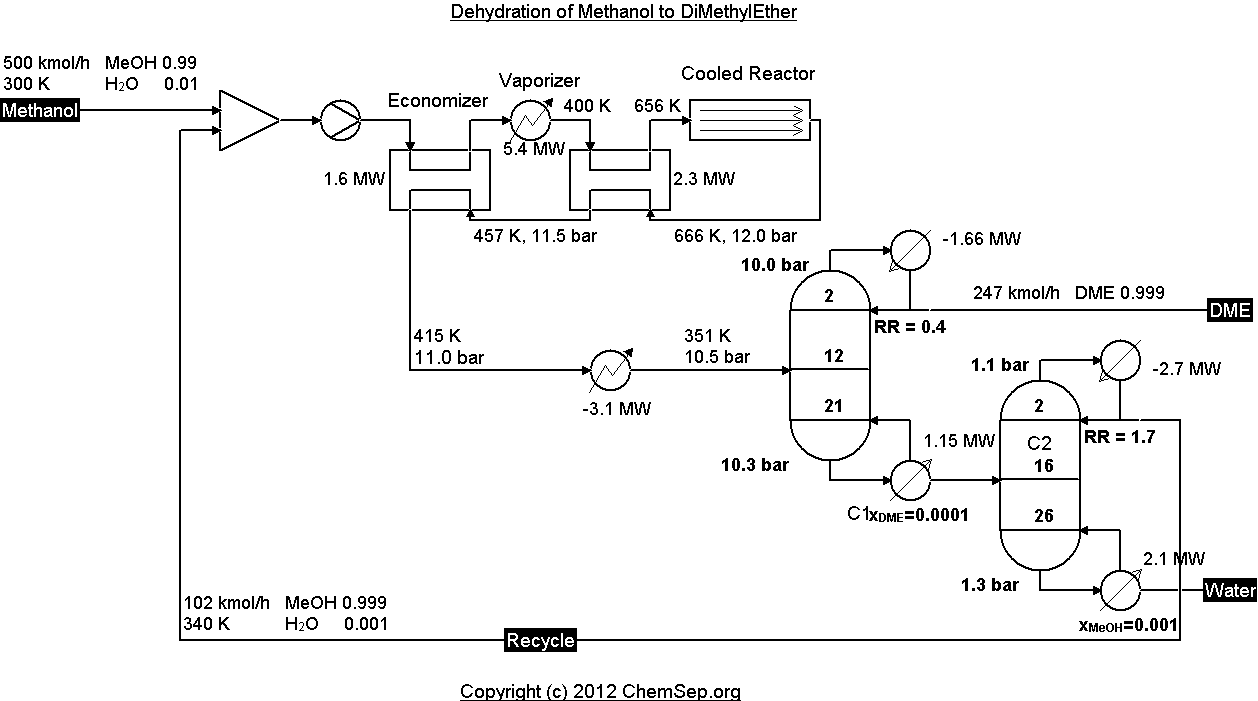 Dehydration of Methanol to produce DiMethylEther (DME) as described by Diemer and Luyben
in Ind.Chem.Eng.Res.Des. Vol. 49 page 12224-12241.
Dehydration of Methanol to produce DiMethylEther (DME) as described by Diemer and Luyben
in Ind.Chem.Eng.Res.Des. Vol. 49 page 12224-12241.
|
| [fsd] [png] |
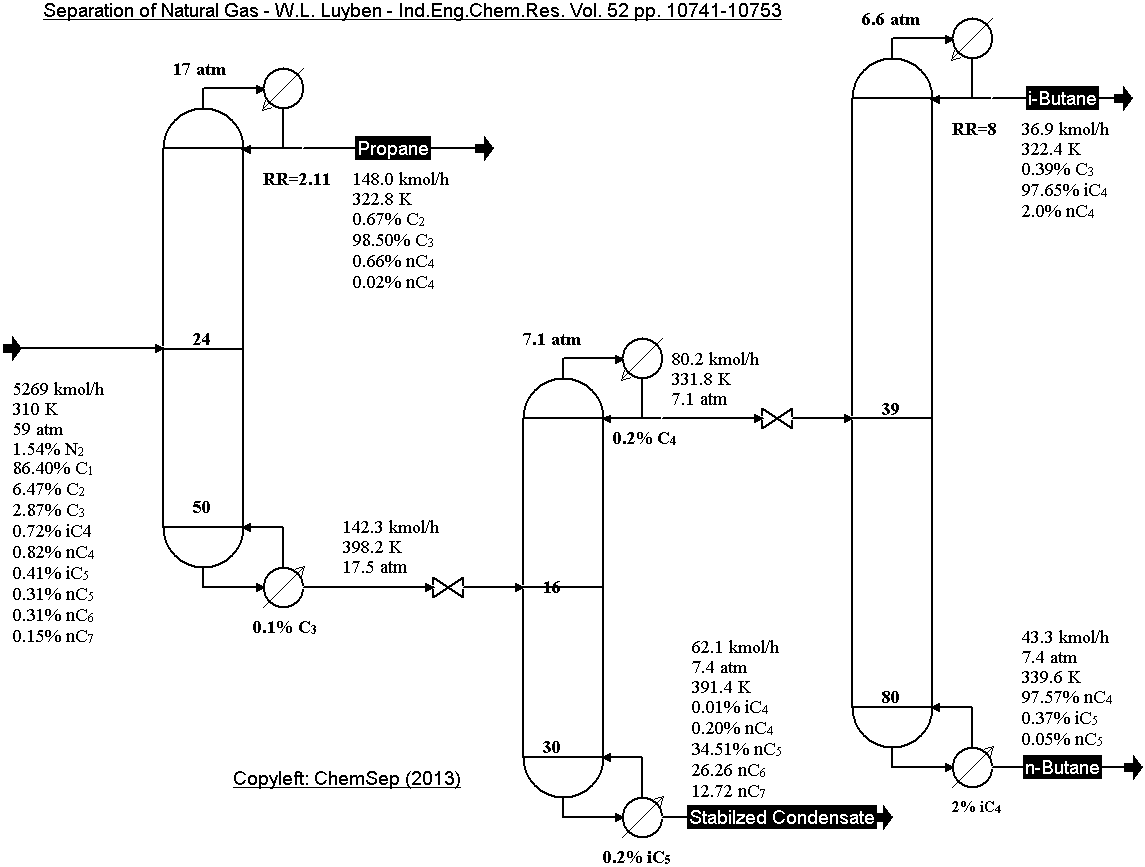
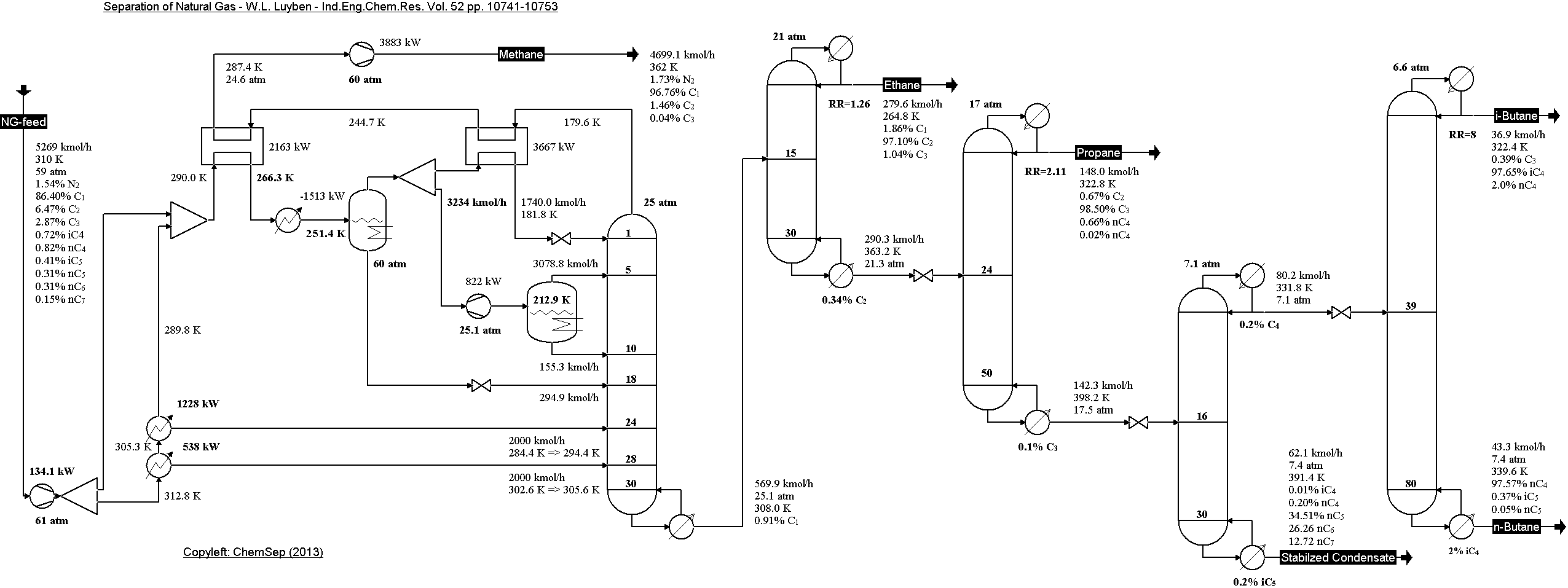 Natural Gas separation train from Luyben in
Ind.Eng.Chem.Res (2013) Vol. 52 pp. 10741-10753.
Natural Gas separation train from Luyben in
Ind.Eng.Chem.Res (2013) Vol. 52 pp. 10741-10753.
|
| [fsd] [png] |
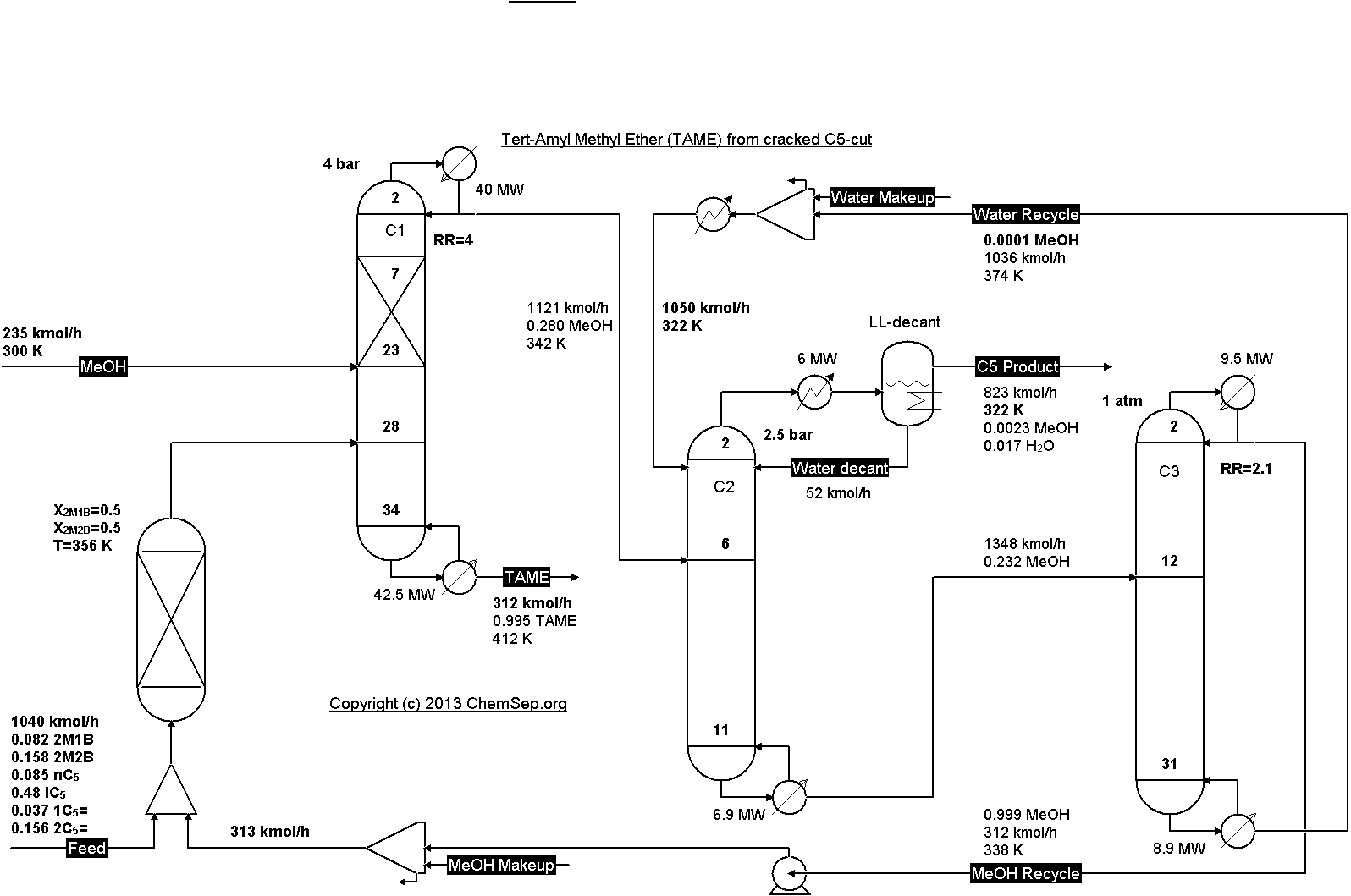 Reactive distillation for producing Tert-Amyl Methyl Ether (TAME) from a cracked C5-cut by Luyben in
Ind.Eng.Chem.Res. (2005) Vol. 44 pp. 5715-5725.
Reactive distillation for producing Tert-Amyl Methyl Ether (TAME) from a cracked C5-cut by Luyben in
Ind.Eng.Chem.Res. (2005) Vol. 44 pp. 5715-5725.
|
| [fsd] [png] [pdf] |
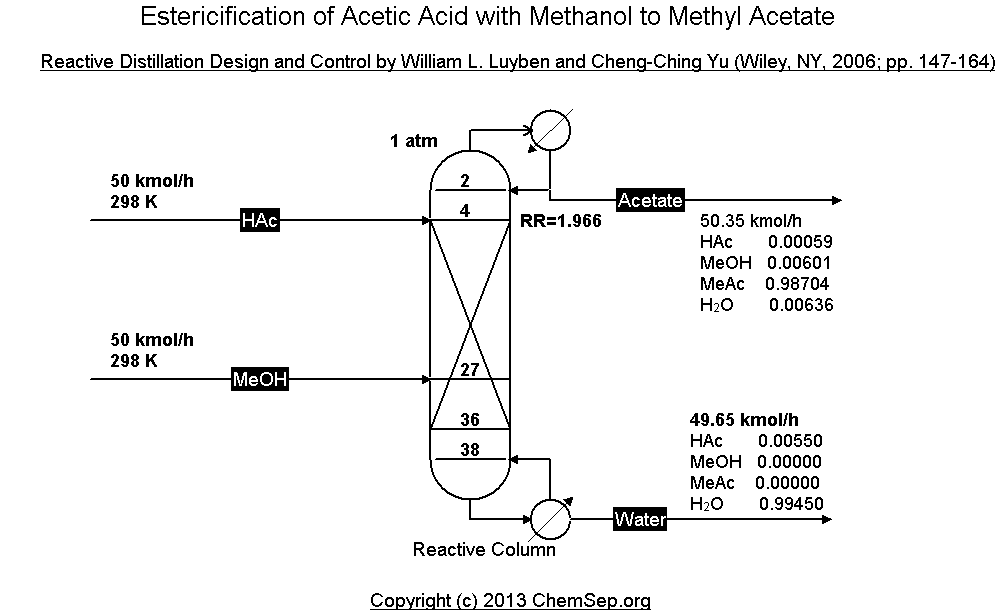 Esterification of Acetic Acid with Methanol to Methyl Acetate
by means of reactive distillation as described in
Reactive Distillation Design and Control by William L. Luyben and Cheng-Ching Yu, Wiley, NY (2006) pp. 147-164.
The PDF file contains the full specification and explanation how to setup this reactive distillation.
Esterification of Acetic Acid with Methanol to Methyl Acetate
by means of reactive distillation as described in
Reactive Distillation Design and Control by William L. Luyben and Cheng-Ching Yu, Wiley, NY (2006) pp. 147-164.
The PDF file contains the full specification and explanation how to setup this reactive distillation.
|
| [fsd] [png] |
 Refinery light ends separations (depropanizer, debutanizer, deisobutanizer) by means of distillation by Luyben in
Ind.Eng.Chem.Res., 52 (2013) pp. 15883-15895.
Refinery light ends separations (depropanizer, debutanizer, deisobutanizer) by means of distillation by Luyben in
Ind.Eng.Chem.Res., 52 (2013) pp. 15883-15895.
|
| [fsd] [png] |
 This case study is a modified version of the 1967 American Institute of Chemical Engineers student contest problem for the
dealkylation of Toluene to Benzene with hydrogen, see "Conceptual Design of Chemical Processes", McGrawHill, 1988,
or J.M Douglas, AIChE J., Vol. 31 (1985) p. 353. It features a gas phase reaction with gas recycle as well as a separation
train with a recycle of unreacted toluene.
This case study is a modified version of the 1967 American Institute of Chemical Engineers student contest problem for the
dealkylation of Toluene to Benzene with hydrogen, see "Conceptual Design of Chemical Processes", McGrawHill, 1988,
or J.M Douglas, AIChE J., Vol. 31 (1985) p. 353. It features a gas phase reaction with gas recycle as well as a separation
train with a recycle of unreacted toluene.
|
| [fsd] [png] |
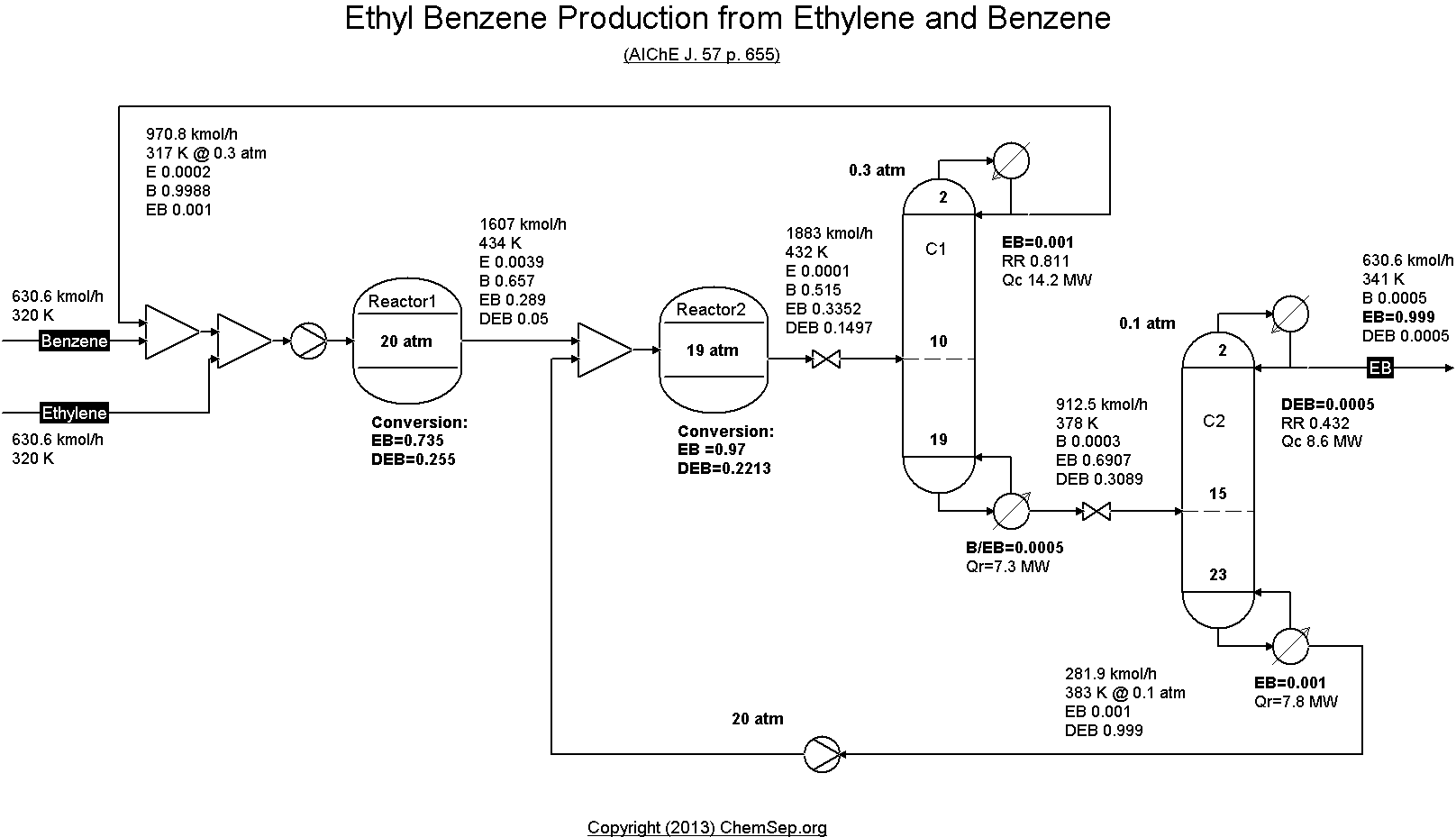 EthylBenzene production from Ethylene and Benzene by Luyben in
AIChE J. Vol. 57 (2011) pp. 655-670.
Note that this flowsheet uses fixed conversion rates in the reactor whereas the original publication uses rate equations.
EthylBenzene production from Ethylene and Benzene by Luyben in
AIChE J. Vol. 57 (2011) pp. 655-670.
Note that this flowsheet uses fixed conversion rates in the reactor whereas the original publication uses rate equations.
|
| [fsd] [png] |
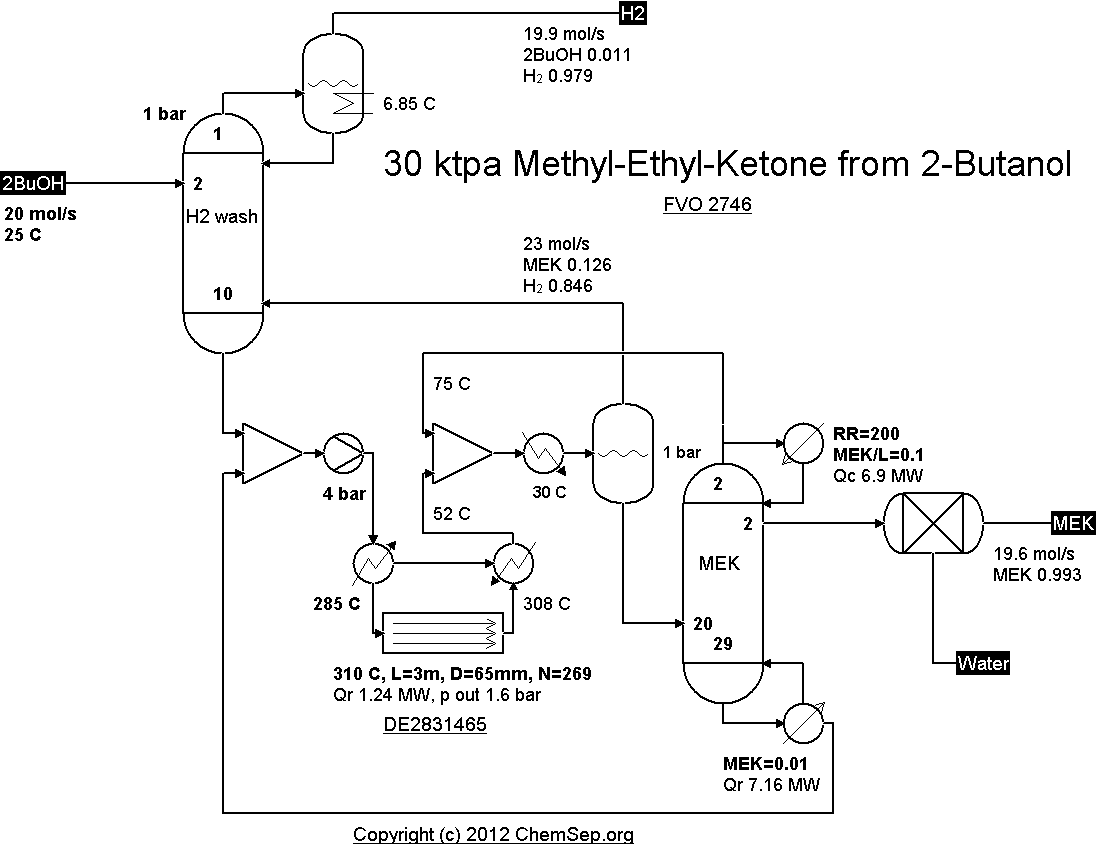 Dehydrogenation of 2-Butanol to Methyl Ethyl Ketone catalyzed by In/MgO as per DE2831465A1 (1978)
Dehydrogenation of 2-Butanol to Methyl Ethyl Ketone catalyzed by In/MgO as per DE2831465A1 (1978)
|
| [fsd] [png] |
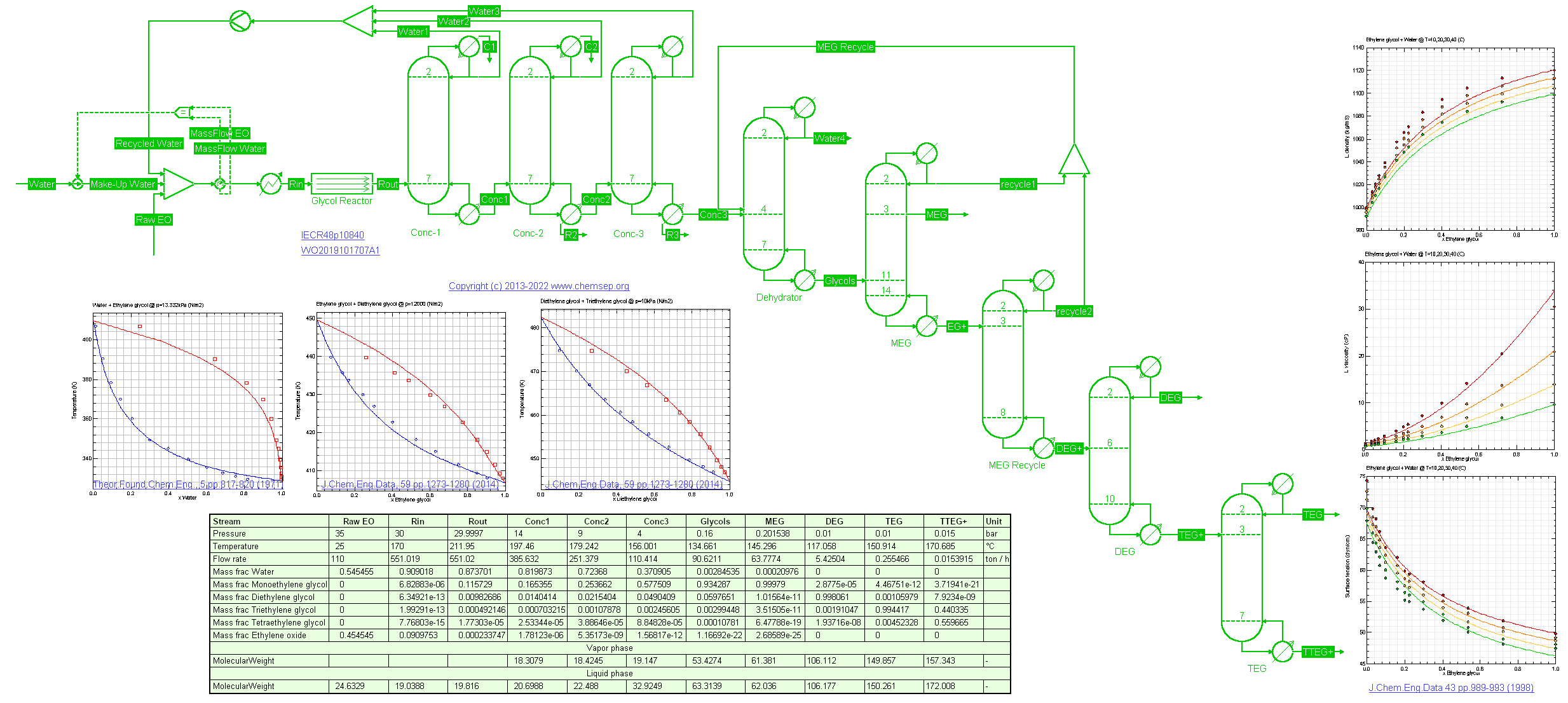 Hydration of Ethylene Oxide to Mono-Ethylene Glycol (MEG) (19/12/2013) using an uncatalyzed reactor at 200 C with kinetics from
Ind.Eng.Chem.Res. 48 (2009) pp. 10840-10844.
Hydration of Ethylene Oxide to Mono-Ethylene Glycol (MEG) (19/12/2013) using an uncatalyzed reactor at 200 C with kinetics from
Ind.Eng.Chem.Res. 48 (2009) pp. 10840-10844.
|
| [fsd] [png] |
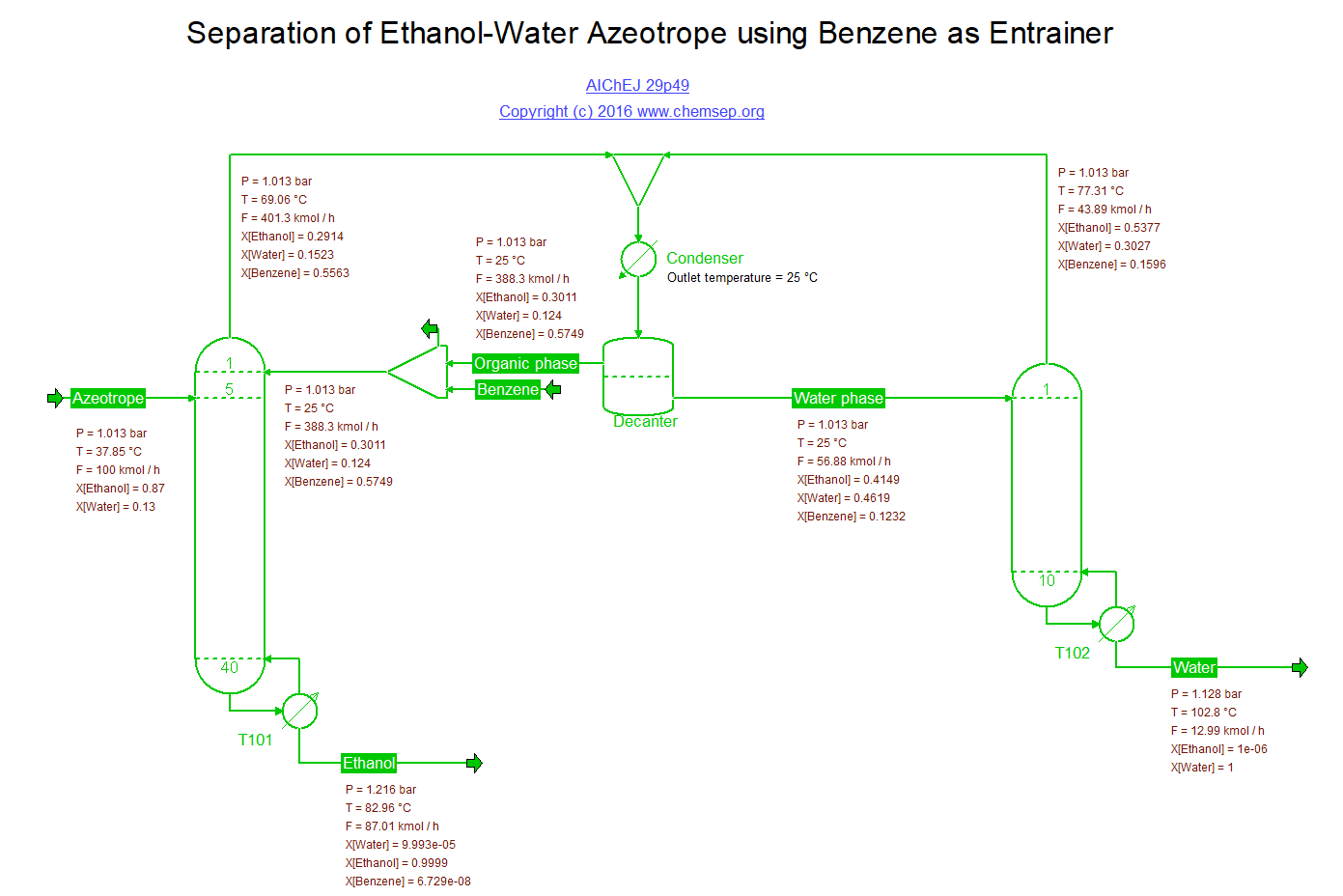 Heterogeneous azeotropic distillation of Ethanol and Water (23/2/2016) inspired by the flowsheet described by G. Prokopakis and W.D. Seider in
AIChE J. 29 p. 49. This separation process model is extremely
sensitive to small changes in the process specifications and also to the parameters used in the thermodynamic model.
Heterogeneous azeotropic distillation of Ethanol and Water (23/2/2016) inspired by the flowsheet described by G. Prokopakis and W.D. Seider in
AIChE J. 29 p. 49. This separation process model is extremely
sensitive to small changes in the process specifications and also to the parameters used in the thermodynamic model.
|
| [fsd] [png] [fsd] [png] |
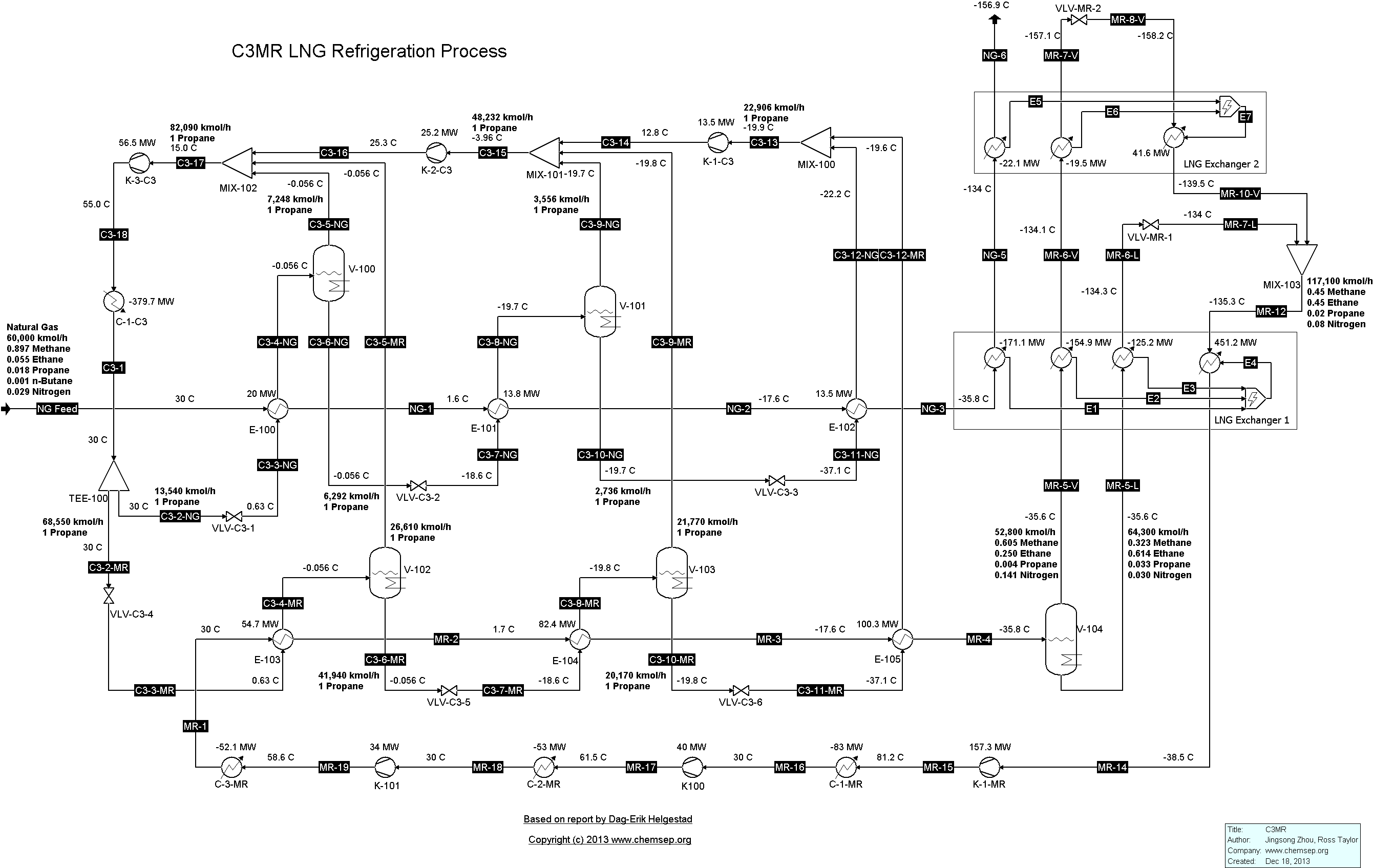
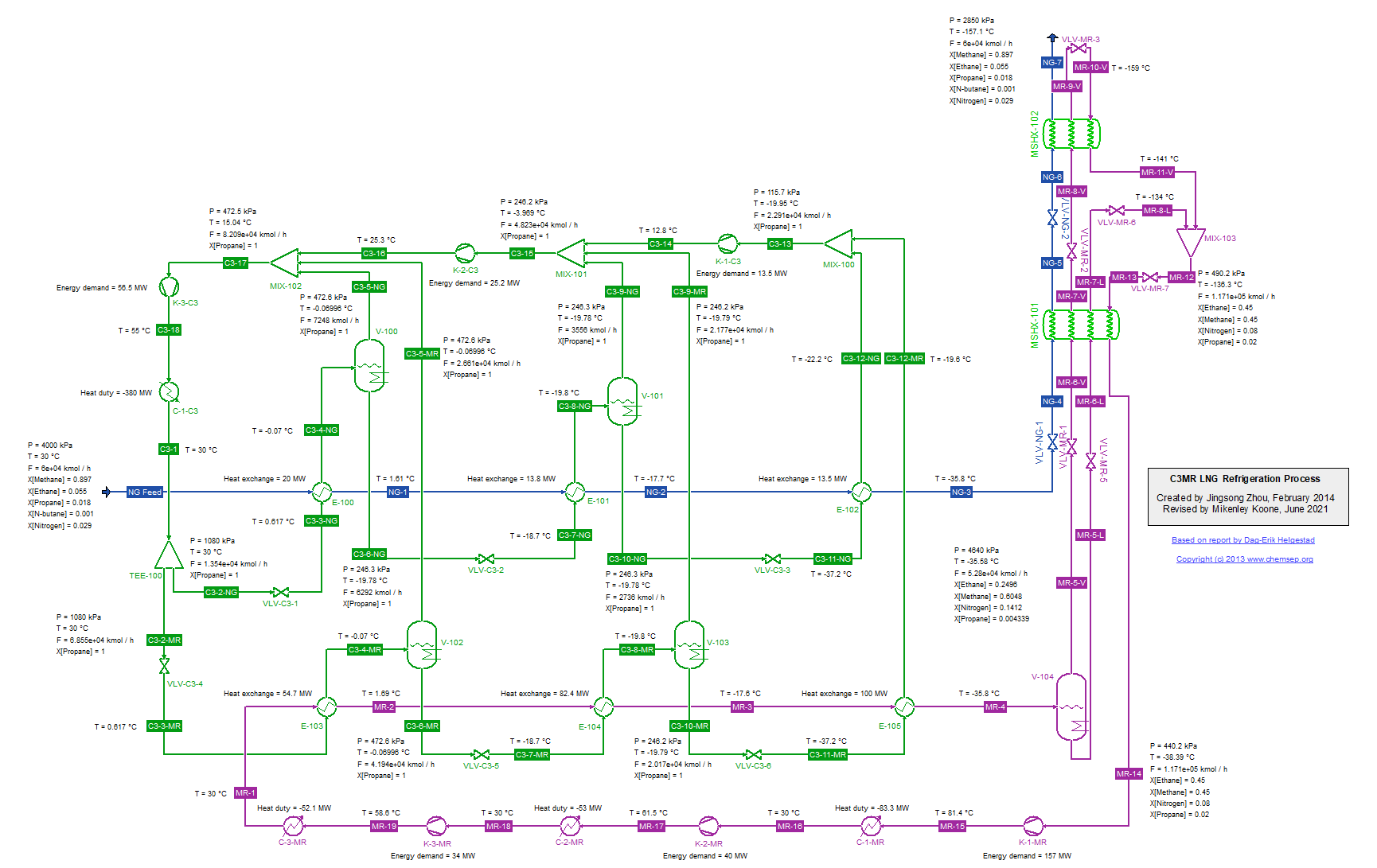 C3MR LNG Refrigeration Cycle for Natural Gas (14/1/2014). This flowsheet was inspired by that given in the report "Modelling and optimization of the C3MR process for
liquefaction of natural gas," by Dag-Erik Helgestad (December 2009).
C3MR LNG Refrigeration Cycle for Natural Gas (14/1/2014). This flowsheet was inspired by that given in the report "Modelling and optimization of the C3MR process for
liquefaction of natural gas," by Dag-Erik Helgestad (December 2009).
|
| [fsd] [png] [fsd] [png] |
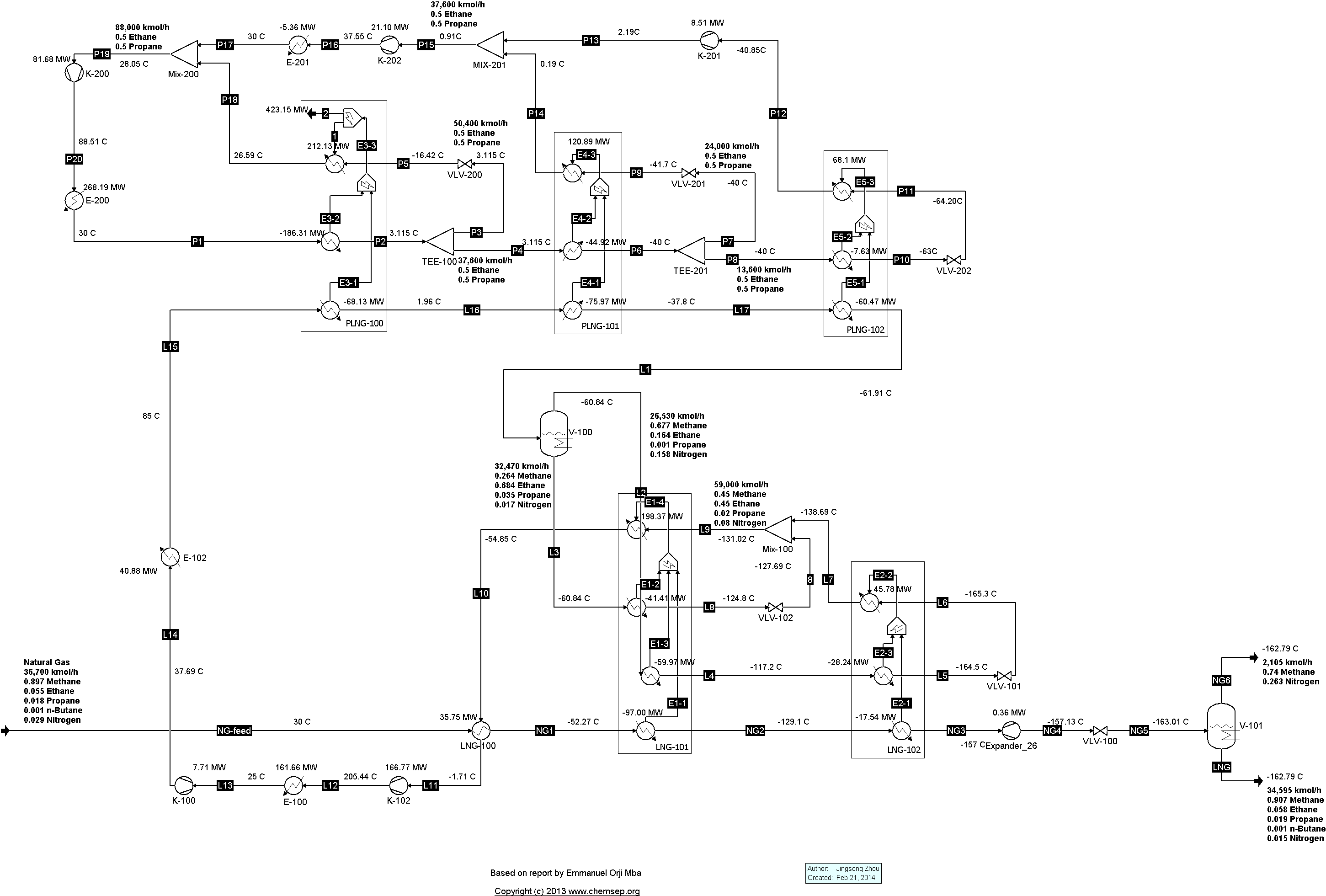
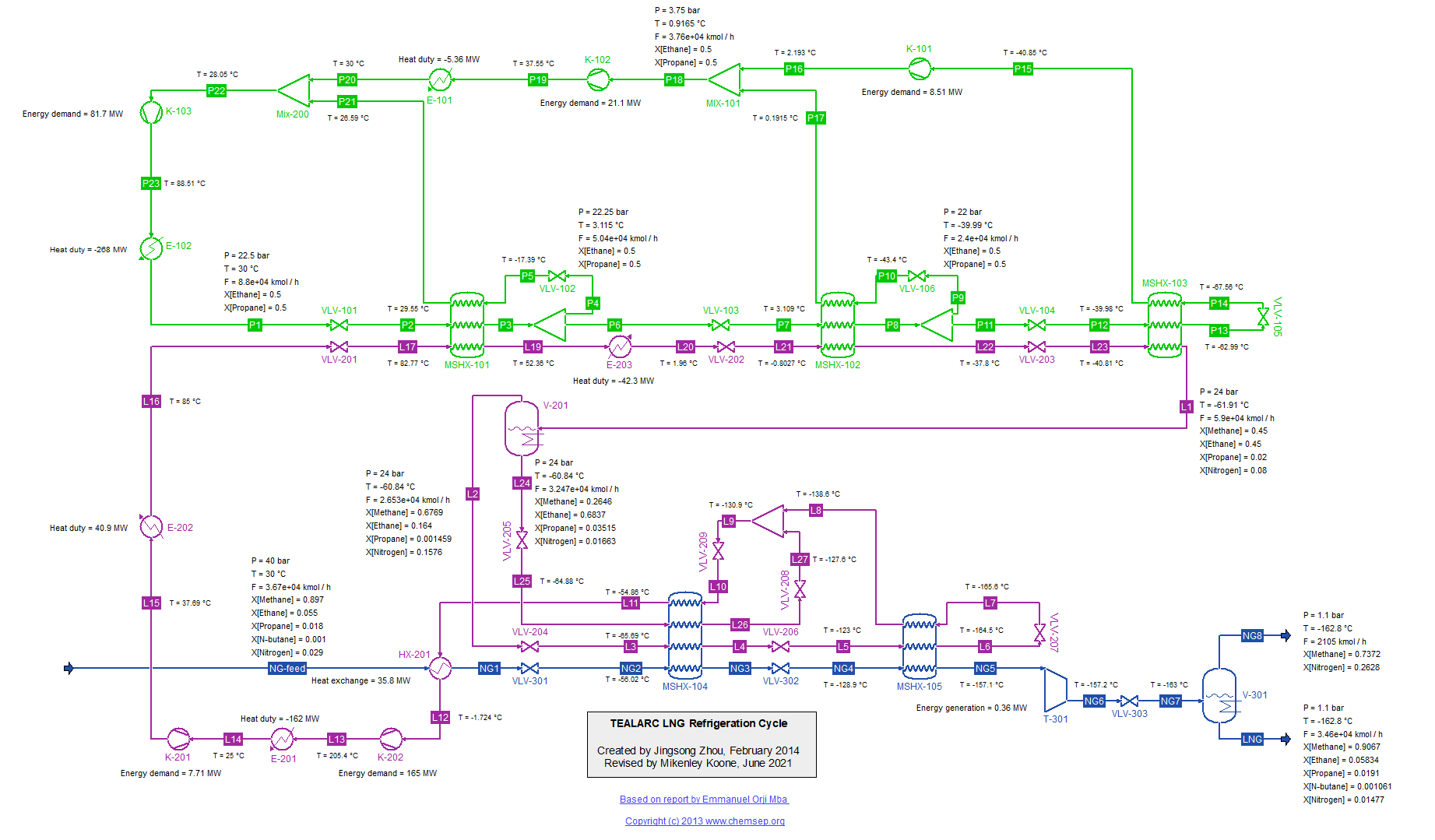 TEALARC LNG Refrigeration Cycle for Natural Gas (21/2/2016). This flowsheet was based on one described in the report
"Simulation, optimal operation and self optimisation of TEALARC LNG plant," by
Emmanuel Orji Mba (December 2009).
TEALARC LNG Refrigeration Cycle for Natural Gas (21/2/2016). This flowsheet was based on one described in the report
"Simulation, optimal operation and self optimisation of TEALARC LNG plant," by
Emmanuel Orji Mba (December 2009).
|
| [fsd] [png] |
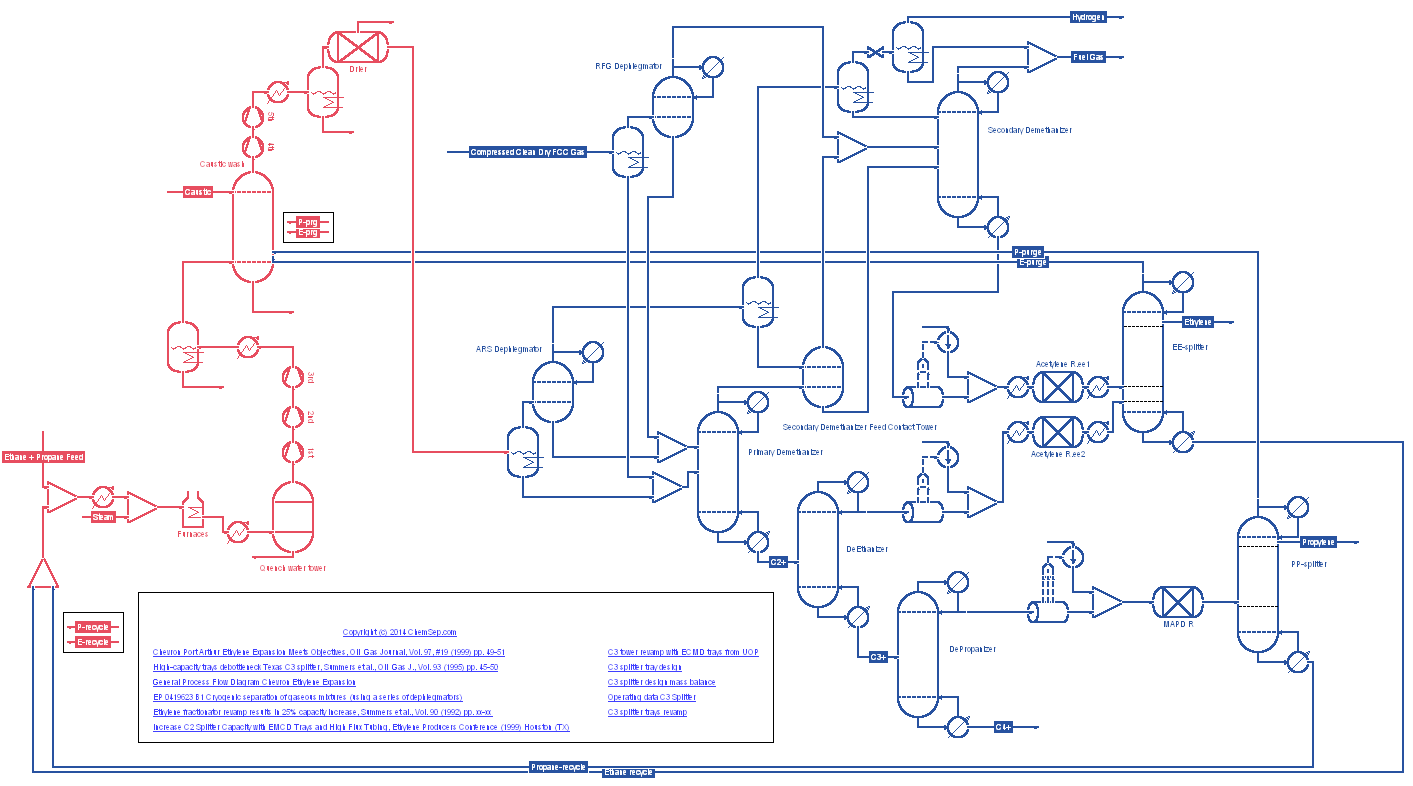 Ethylene Cracker with high purity separation train using UOP Multi-Downomer trays based on the debottlenecking of the EE splitter and
the PP splitter of the
Port Arthur (TX) Chevron Ethylene Cracker.
"Stone and Webster's ARS technology was implemented in Chevron's ARS and refinery-gas dephlegmator coldboxes during the revamp in 1997.
Chevron Chemical Co. LLC's Port Arthur, Tex., ethylene unit (EU-1544) was expanded from 1.0 billion lb/year to 1.7 billion lb/year.".
Ethylene Cracker with high purity separation train using UOP Multi-Downomer trays based on the debottlenecking of the EE splitter and
the PP splitter of the
Port Arthur (TX) Chevron Ethylene Cracker.
"Stone and Webster's ARS technology was implemented in Chevron's ARS and refinery-gas dephlegmator coldboxes during the revamp in 1997.
Chevron Chemical Co. LLC's Port Arthur, Tex., ethylene unit (EU-1544) was expanded from 1.0 billion lb/year to 1.7 billion lb/year.".
|
| [fsd] [png] |
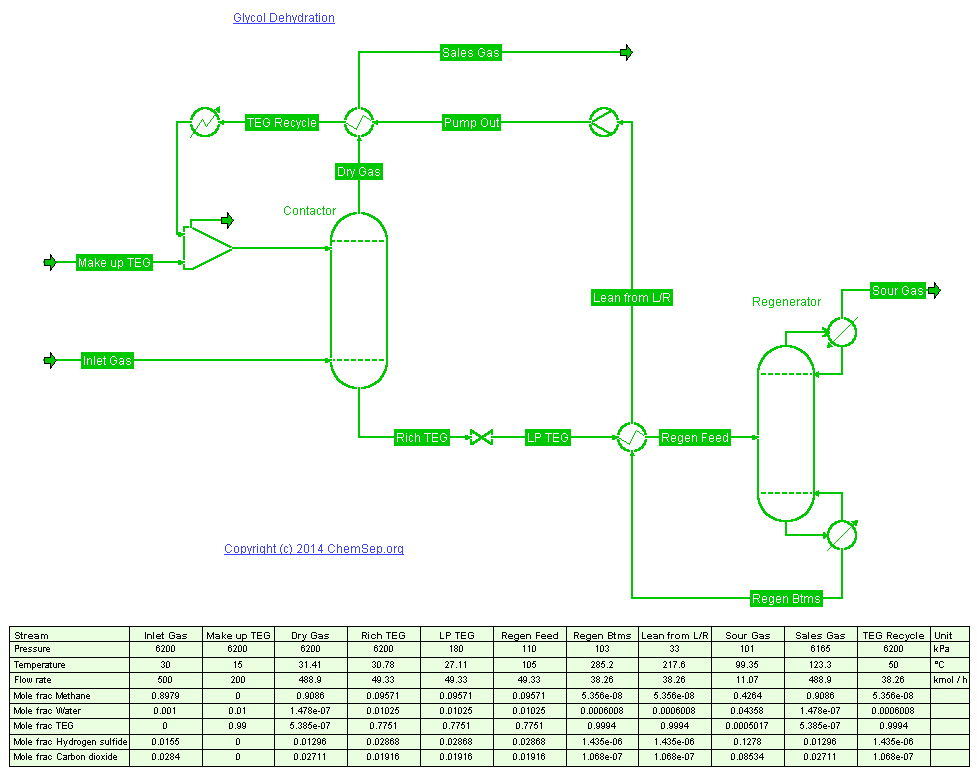 Drying of Natural Gas using TEG.
Water vapor is the most common undesirable impurity in natural gas streams as it can form liquid or hydrates (solids) when the gas is compressed
or cooled. Liquid water accelerates corrosion and ice or hydrates can plug valves, fittings, and even gas lines.
To prevent such difficulties in transmission lines, the gas is dehydrated, usually using Glycols, because they are stable for thermal and chemical
degradation, and their hygroscopic nature makes for an efficient water removal.
In this 2010 example from the CHBE 323 course by professor Jeffrey Heys TriEthyleneGlycol (TEG) is used to dry a natural gas stream at high pressure
(62 bar) with a 30 to 1 mass ratio at 50 deg C. The TEG regenerator removes water to a concentration of less than 1 wt percent water.
The VLE was modelled using the Peng-Robinson EOS where Binary Interaction Parameters (BIPs) for TEG/Water and Water/Methane need to be fit,
to get an accurate estimate for the amount of gas that flashes off from the rich solvent.
Drying of Natural Gas using TEG.
Water vapor is the most common undesirable impurity in natural gas streams as it can form liquid or hydrates (solids) when the gas is compressed
or cooled. Liquid water accelerates corrosion and ice or hydrates can plug valves, fittings, and even gas lines.
To prevent such difficulties in transmission lines, the gas is dehydrated, usually using Glycols, because they are stable for thermal and chemical
degradation, and their hygroscopic nature makes for an efficient water removal.
In this 2010 example from the CHBE 323 course by professor Jeffrey Heys TriEthyleneGlycol (TEG) is used to dry a natural gas stream at high pressure
(62 bar) with a 30 to 1 mass ratio at 50 deg C. The TEG regenerator removes water to a concentration of less than 1 wt percent water.
The VLE was modelled using the Peng-Robinson EOS where Binary Interaction Parameters (BIPs) for TEG/Water and Water/Methane need to be fit,
to get an accurate estimate for the amount of gas that flashes off from the rich solvent.
|
|
[fsd][png] [fsd][png] [fsd][png] [fsd][png] [fsd][png] |
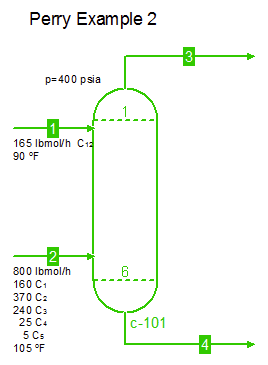
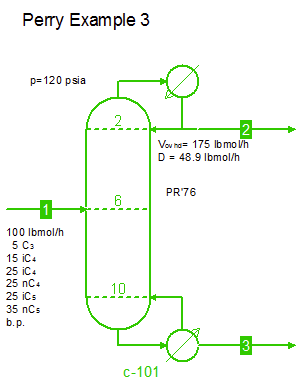
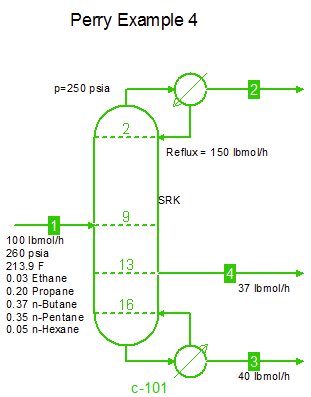
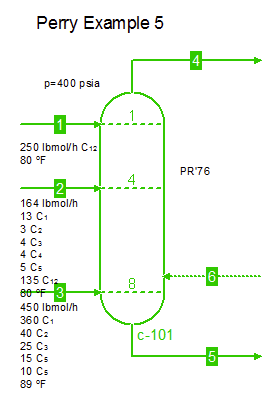
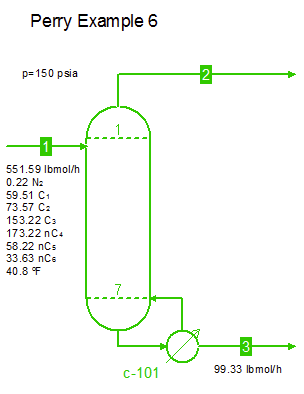 Examples from Chapter 13 Distillation of Perry's Chemical Engineers' Handbook:
Examples from Chapter 13 Distillation of Perry's Chemical Engineers' Handbook:2) Simple absorber for Butane and Pentane recovery from process gas using absorbent oil (here simulated with n-Dodecane), 3) Simple two cut splitter separating Butane and Pentane, 4) Three cut splitter with side-draw that creates a sloppy Butane cut, 5) Two-step absorber with internal cooling to maximize LPG recovery, and 6) Reboiled stripper to remove light gases (N2, C1-C3) from heavier compounds. |
| [fsd] [png] |
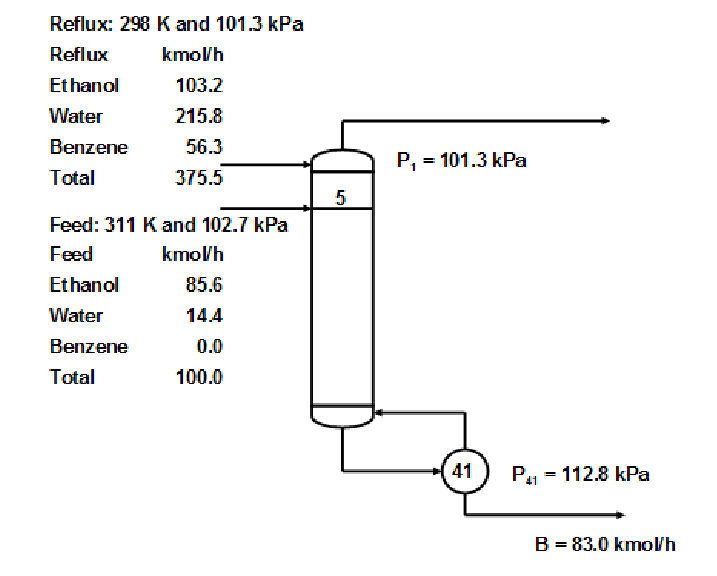 Ethanol Water separation with Benzene exhibiting multiplicity
This example is one of the most famous in the entire literature on distillation column modeling
having been studied, in one form or another, by many investigators including
Magnussen et al. I.Chem.E.Symp.Series, 56 (1979),
Prokopakis and Seider AIChE J., 29, 49 (1983),
and Venkataraman and Lucia Comput.Chem.Engng., 12, 55 (1988).
The column simulated here is adapted from the work of Prokopakis and Seider.
Ethanol Water separation with Benzene exhibiting multiplicity
This example is one of the most famous in the entire literature on distillation column modeling
having been studied, in one form or another, by many investigators including
Magnussen et al. I.Chem.E.Symp.Series, 56 (1979),
Prokopakis and Seider AIChE J., 29, 49 (1983),
and Venkataraman and Lucia Comput.Chem.Engng., 12, 55 (1988).
The column simulated here is adapted from the work of Prokopakis and Seider. . .
|
| [fsd] [png] |
 Solvents recovery line-up based on Sep.Purif.Technol. 169 (2016) pp. 66-77
combining azeotropic distillation with pressure swing distillation into a three column line-up for recovery of Acrylonitril, Methanol, and Benzene. This mixture forms
multiple azeotropes and its triangular diagram has several distillation boundaries at atmospheric pressure. The feasibility of the process was
confirmed using rigorous steady-state simulations. This 3 column line-up is the most optimal column sequence in a global optimization to separate the azeotropic mixture.
Solvents recovery line-up based on Sep.Purif.Technol. 169 (2016) pp. 66-77
combining azeotropic distillation with pressure swing distillation into a three column line-up for recovery of Acrylonitril, Methanol, and Benzene. This mixture forms
multiple azeotropes and its triangular diagram has several distillation boundaries at atmospheric pressure. The feasibility of the process was
confirmed using rigorous steady-state simulations. This 3 column line-up is the most optimal column sequence in a global optimization to separate the azeotropic mixture.
|
| [fsd] [png] |
 Acetone is produced via several alternative processes, one of which is the Acetone Process via Dehydrogenation of 2-Propanol (IPA).
This endothermic gas-phase reaction converts IPA to acetone and hydrogen. The process has two distillation columns and an absorber
column in which a water stream is used to recover acetone. In Ind.Eng.Chem.Res. Vol. 50 pp. 1206-1218 (2011)
Luyben showed that operating the absorber at an elevated pressure reduced Acetone losses but increases vent losses and raises the required temperature and cost of the
vaporizer heat source. It also adversely affects the reaction kinetics because the reaction is non-equimolar and conversion decreases with increasing pressure.
As such, a higher reactor temperature is required to achieve the desired conversion. The paper proposed the economically optimum design.
Acetone is produced via several alternative processes, one of which is the Acetone Process via Dehydrogenation of 2-Propanol (IPA).
This endothermic gas-phase reaction converts IPA to acetone and hydrogen. The process has two distillation columns and an absorber
column in which a water stream is used to recover acetone. In Ind.Eng.Chem.Res. Vol. 50 pp. 1206-1218 (2011)
Luyben showed that operating the absorber at an elevated pressure reduced Acetone losses but increases vent losses and raises the required temperature and cost of the
vaporizer heat source. It also adversely affects the reaction kinetics because the reaction is non-equimolar and conversion decreases with increasing pressure.
As such, a higher reactor temperature is required to achieve the desired conversion. The paper proposed the economically optimum design.
|
| [fsd] [png] |
 The Energy Efficient Hybrid Separation process for Acetic Acid purification is based on
Ind. Eng. Chem. Res. Vol. 45, pp. 8319-8328 (2006),
a paper discussing strategies that combine one or more separation techniques with distillation where energy efficiency is studied using the novel concept of shortest separation lines.
Such hybrid separation schemes include extraction followed by distillation, reactive distillation, adsorption/distillation, and others.
The Energy Efficient Hybrid Separation process for Acetic Acid purification is based on
Ind. Eng. Chem. Res. Vol. 45, pp. 8319-8328 (2006),
a paper discussing strategies that combine one or more separation techniques with distillation where energy efficiency is studied using the novel concept of shortest separation lines.
Such hybrid separation schemes include extraction followed by distillation, reactive distillation, adsorption/distillation, and others.
|
| [fsd] [png] |
 Cyclohexane can be produced by the Hydrogenation of Benzene by the ARCO Technology Inc. process as described in Hydrocarbon Processing, November (1977) p. 143. This process has been
replaced by the more efficient and economic reactive distillation hydrogenation process from CDtech (US6187980).
Cyclohexane can be produced by the Hydrogenation of Benzene by the ARCO Technology Inc. process as described in Hydrocarbon Processing, November (1977) p. 143. This process has been
replaced by the more efficient and economic reactive distillation hydrogenation process from CDtech (US6187980).
|
| [fsd] [png] |
 The Styrene process from EthylBenzene is based on the Vasudevan design in Ind. Eng. Chem. Res. Vol. 48, pp. 10941 (2009),
Figure 15.1. This paper discusses an improvement design over the Styrene plant in "Plant-Wide Process Control" by Luyben et al. (McGraw-Hill, NY, 1998).
The Styrene process from EthylBenzene is based on the Vasudevan design in Ind. Eng. Chem. Res. Vol. 48, pp. 10941 (2009),
Figure 15.1. This paper discusses an improvement design over the Styrene plant in "Plant-Wide Process Control" by Luyben et al. (McGraw-Hill, NY, 1998).
|
All your suggestions are welcome, send them to Harry Kooijman.
Copyright © Wed Nov 12 01:08:25 2025 ChemSep